ChemicalBook > CAS DataBase List > Cefotiam
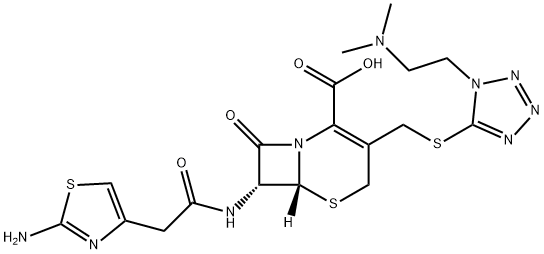
Cefotiam
Cefotiam
- CAS No.61622-34-2
- Chemical Name:Cefotiam
- CBNumber:CB7367629
- Molecular Formula:C18H23N9O4S3
- Formula Weight:525.63
- MOL File:61622-34-2.mol
Cefotiam Property
- Boiling point 843℃
- Density 1.80±0.1 g/cm3(Predicted)
- RTECS XI0366000
- Flash point >110°(230°F)
- storage temp. 2-8°C
- pka 2.57±0.50(Predicted)
- Water Solubility Soluble
- CAS DataBase Reference 61622-34-2(CAS DataBase Reference)
- FDA UNII 91W6Z2N718
- ATC code J01DC07
-
Symbol(GHS)

- Signal wordDanger
- Hazard statements H317-H334
- Precautionary statements P261-P272-P280-P302+P352-P333+P313-P321-P363-P501-P261-P285-P304+P341-P342+P311-P501
Cefotiam Chemical Properties,Usage,Production
- Originator Cefotiam,Qingdao Ftz United International Inc.
- Uses Antibacterial
- Definition ChEBI: A cephalosporin with ({1-[2-(dimethylamino)ethyl]-1H-tetrazol-5-yl}sulfanyl)methyl and (2-amino-1,3-thiazol-4-yl)acetamido substituents at positions 3 and 7, respectively, of the cephem skeleton. A third generation beta-lactam cephalospo in antibiotic, it is active against a broad spectrum of both Gram positive and Gram negative bacteria.
-
Manufacturing Process
2 mmol of 7-acetamindocephalosporinic acid in phosphate buffer (pH 7), 2
mmol of 1-(2-dimethylaminoethyl-1H-tetrazol-5-thiol and 2.2 mmol of sodium
hydrogen carbonate are mixed and resulting solution was stirred at 60°-65°C
for 16 hours. After cooling the mixture was adjusted pH 3.6 and hydrochloride
of NH2OH was added to give 7-amino-3-[2-(2-dimethylaminoethyl)-2H-tetrazol-5-ylsulfanylmethyl]-8-oxo-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-
carboxylic acid.
0.4 g (2 mmol) of 2-(2-aminothiazol-4-yl)acetic acid hydrochloride in 10 ml of dimethylformamide are dissolved, 0.25 g (2.2 mmol) of N-hydroxysuccinimide and 0.412 g (2 mmol) of dicyclohexylcarbodiimide and the solution is allowed to stand at room temperature for 3 hours. The reaction mixture is subjected to filtration under suction to remove the precipitate of N,N'-dicyclohexylurea. The filtrate is added at a stroke to a solution of 2 mmol of above prepared 7- amino-3-[2-(2-dimethylaminoethyl)-2H-tetrazol-5-ylsulfanylmethyl]-8-oxo-5- thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 0.404 g (4 mmol) of triethylamine in 20 ml of dichloromethane and the mixed solution is stirred for 24 hours at room temperature. The solvent is distilled off under reduced pressure and the residue is adjusted pH from to 6 to 7 by adding a 10% aqueous solution of sodium hydrogen carbonate. The resultant solution is chromatographed on column of polystyrene resin (Amberlite XAD-2) and developed with water. The fractions containing the desired product are pooled and freeze-dried to obtain the title product. - brand name Ceradon (Takeda).
- Therapeutic Function Antibiotic
-
Antimicrobial activity
A semisynthetic cephalosporin formulated as the dihydrochloride
for injection and as a prodrug ester, cefotiam hexetil, for
oral administration. Activity is similar to that of cefuroxime,
but it is somewhat more active against a range of enterobacteria
.
A 30-min intravenous infusion of the dihydrochloride produces a peak serum concentration of 35 mg/L; the corresponding concentration after a 1 g intramuscular dose is 17 mg/L. Oral absorption of the hexetil ester is around 65%. Food delays absorption of the ester. The plasma half-life is 0.6–1.1 h. Around 40% is bound to plasma protein. Urinary excretion is almost complete 4 h after the end of intravenous infusion, but only 50–67% is recovered unchanged; there is substantial non-renal elimination and some evidence of saturation of renal tubular excretion at doses above 2 g. In anuria the plasma elimination half-life rises to 13 h and plasma and renal clearances parallel creatinine clearance. A small amount is excreted in bile. In patients with cholelithiasis given 0.5 or 1 g intravenously, mean concentrations in gallbladder bile and gallbladder wall 30 min after the dose were around 17 and 32 mg/L, respectively. In patients with normal liver function, hepatic bile concentrations can exceed 1 g/L.
It is generally well tolerated and has been used successfully to treat lower respiratory infections, skin and soft-tissue infection. It is not widely used, but is available in Japan and some other countries.
Cefotiam Preparation Products And Raw materials
Raw materials
Preparation Products
Global(95)Suppliers
-
Supplier:
Shaanxi TNJONE Pharmaceutical Co., Ltd
- Tel:+86-17396673057
- Email:linda@tnjone.com
- Country:China
- ProdList:1143
- Advantage:58
-
Supplier:
Shaanxi Xianhe Biotech Co., Ltd
- Tel: +8617709210191
- Email:Jerry@xhobio.com
- Country:China
- ProdList:884
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29858
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-89586680<br/>+86-18192503167
- Email:1026@dideu.com
- Country:China
- ProdList:7859
- Advantage:58
-
Supplier:
AFINE CHEMICALS LIMITED
- Tel:+86-0571-85134551
- Email:sales@afinechem.com
- Country:China
- ProdList:15395
- Advantage:58
-
Supplier:
Zhejiang J&C Biological Technology Co.,Limited
- Tel:+1-2135480471<br/>+1-2135480471
- Email:sales@sarms4muscle.com
- Country:China
- ProdList:10473
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:
- Email:support@targetmol.com
- Country:United States
- ProdList:38630
- Advantage:58
-
Supplier:
GIHI CHEMICALS CO.,LIMITED
- Tel: +8618058761490
- Email:info@gihichemicals.com
- Country:China
- ProdList:49984
- Advantage:58
Related articles
61622-34-2, CefotiamRelated Search:
- 2-(2-Aminothiazol-4-yl)glyoxylic acid N-ACETYL-L-TYROSINE ETHYL ESTER 4-Dimethylaminobenzoic acid 4-Dimethylaminopyridine Glycine Acetyl chloride 4-Dimethylaminobenzaldehyde 3-Dimethylaminopropylamine 6-Aminocaproic acid Acetylacetone ALTRENOGEST Acetyl coenzyme A sodium salt Tris(hydroxymethyl)aminomethane CEFOTIAM 2HCL,cefotiam hydrochloride 2-Dimethylaminoethanol CefotiaM Hexetil Hydrochloride 2-(2-aminothiazole-4-yl)acetic acid hydrochloride (intermediate of cefotiam) cefotiam hexetil hydrochloride
- 抑制剂
- 医药原料药
- 化药杂质-头孢替安
- 医药化工类
- 对照品-杂质对照品
- 药物
- 抗生素
- β-内酰胺类抗生素
- 医药中间体
- 化合物 CEFOTIAM
- 7-[[(2-氨基-4-噻唑基)乙酰基]氨基]-3-[[[1-[(2-(二甲氨基)乙基]-1H-四唑-5-基]硫代甲基]-8-氧代-5-硫杂-1-氮杂双环[4.2.0]辛-2-烯-2-羧酸
- 头孢替胺盐酸盐
- (6R-反式)-7-[[(2-氨基-4-噻唑基)乙酰基]氨基]-3-[[[1-[2-(二甲基氨基)乙基]-1H-四唑-5-基]硫代]甲基]-8-氧代-5-硫杂-1-氮杂二环[4.2.0]辛酸-2-烯-2-羧酸
- 头孢替胺
- 头孢替安
- 61622-34-2
- Cefotiam USP/EP/BP
- Cefotiam Crude N-acetylneuraminic
- 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[2-(2-aMino-4-thiazolyl)acetyl]aMino]-3-[[[1-[2-(diMethylaMino)ethyl]-1H-tetrazol-5-yl]thio]Methyl]-8-oxo-, (6R,7R)-
- (6R,7R)-7α-[(2-Amino-4-thiazolyl)acetylamino]-3-[[[1-[2-(dimethylamino)ethyl]-1H-tetrazol-5-yl]thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
- Texodil
- Taketiam
- Pansporin:Pansporine
- Pansporin T
- (6R-trans)-7-[[(2-Amino-4-thiazlyl)acetyl]amino]-3-[[[1-[2-(dimethylamino)ethyl]-1H-tetrazol-5-y1]thio]methyl]-8-oxo-5-thia-1-azabi-cyclo[4.2.0]oct-2-ene-2-carboxylic acid
- Cefotiam (base and/or unspecified salts)
- SCE 963
- 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2-amino-4-thiazolyl)acetyl]amino]-3-[[[1-[2-(dimethylamino)ethyl]-1H-tetrazol-5-yl]thio]methyl]-8-oxo-, (6R-trans)-
- 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 7-[[(2-amino-4-thiazolyl)acetyl]amino]-3-[[[1-[2-(dimethylamino)ethyl]-1H-tetrazol-5-yl]thio]methyl]-8-oxo-, (6R,7R)-
- CEFOTIAM HCL
- CEFOTIAM
- cgp14221e
- )acetyl)amino)-3-(((1-(2-(dimethylamino)ethyl)-1h-tetrazol-5-yl)thio)methyl)-8
- (6r-trans)--oxo
- 8-[2-(2-amino-1,3-thiazol-4-yl)acetyl]amino-4-[[1-(2-dimethylaminoethyl)tetrazol-5-yl]sulfanylmethyl]-7-oxo-2-thia-6-azabicyclo[4.2.0]oct-4-ene-5-carboxylic acid