ChemicalBook > CAS DataBase List > CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl ester
CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl ester
CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl ester
- CAS No.917342-29-1
- Chemical Name:CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl ester
- CBNumber:CB72668724
- Molecular Formula:C13H25NO3
- Formula Weight:243.34
- MOL File:917342-29-1.mol
CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl ester Property
- Boiling point 366.0±11.0 °C(Predicted)
- Density 1.03±0.1 g/cm3(Predicted)
- storage temp. Sealed in dry,Room Temperature
- pka 12.56±0.40(Predicted)
- InChI InChI=1S/C13H25NO3/c1-13(2,3)17-12(16)14-11-6-4-10(5-7-11)8-9-15/h10-11,15H,4-9H2,1-3H3,(H,14,16)/t10-,11-
- InChIKey KTNFSGIXLVLQNK-XYPYZODXSA-N
- SMILES C(OC(C)(C)C)(=O)N[C@@H]1CC[C@@H](CCO)CC1
Safety
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl ester Chemical Properties,Usage,Production
- Uses trans-1-(Boc-amino)-4-(2-hydroxyethyl)cyclohexane can be used for preparing quinolinones and analogs for treating multi-drug resistant bacterial infections. It can also be used for preparing the antipsychotic drug Cariprazine.
- Uses trans-1-(Boc-amino)-4-(2-hydroxyethyl)cyclohexane can be used for preparing quinolinones and analogs for treating multi-drug resistant bacterial infections. It can also be used for preparing the antipsychotic drug Cariprazine.
- Application (trans-4-Hydroxymethylcyclohexyl)carbamic Acid tert-Butyl Ester is used to prepare C-2 hydroxyethyl imidazopyrrolo pyridines as JAK1 inhibitors. It is also used to prepare Mer kinase inhibitors for treatment of pediatric acute lymphoblastic leukemia.
-
Synthesis
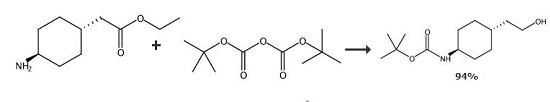
An 500 ml four-necked flask is charged with 40 g (0,18 mol) of trans 2-[1-(4-amino]- cyclohexyl)-acetic acid ethyl ester hydrochloride and 160 ml of dichloromethane. To the resulting suspension 18,2 g (0,18 mol) of triethylamine is added. The reaction mixture is cooled to a temperature between 8-10°C and a solution of 40,0 g (0,185 mol) of di(tert-butyl)dicarbonate in 100 ml of dichloromethane is added for 1 hour with stirring under nitrogen. Then the reaction mixture is allowed to warm to a temperature between 22-25°C and stirred until the reaction proceeds. After completion of the reaction 100 g of 5% aqueous sodium carbonate is added and the phases are separated. The organic layer is extracted with 50 ml of water and after separation the organic layer is dried under Na2SO4 and the filtrate is concentrated in vacuum. The trans 2-{1-[4-(N-tert-butoxycarbonyl)-amino]-cyclohexyl}-acetic acid ethyl ester obtained is dissolved in 460 ml of tetrahydrofurane then 13,68 g (0,36 mol) of sodium borohydride is added at 25°C under nitrogen. With stirring, to the reaction mixture a solution of 24,0 g (0,18 mol) of aluminium chloride in 250 ml abs. tetrahydrofurane is added dropwise at a temperature between 18-22°C for 1 hour under nitrogen then the mixture is stirred for additional 2 hours. After completion of the reaction the mixture is cooled to a temperature between 5-10°C and 650 ml of water and 600 ml of toluene are added. Then the pH was adjusted to 3-4 by adding 40-45 ml of concentrated hydrochloric acid and the stirring was continued at a temperature between 20-25°C for 1 hour. The phases are separated, the aqueous layer is extracted with 50 ml of toluene and the combined organic layers are washed with 3×150 ml of water and dried in vacuum. In this manner 41,1 g of title compound was obtained. Yield: 94% 20. Melting point: 101-103°C.
CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl ester Preparation Products And Raw materials
Raw materials
Preparation Products
Global(114)Suppliers
-
Supplier:
Shanghai Yongzeng Technology Co.,Ltd
- Tel: +8613818947575
- Email:sales@yongzchem.com
- Country:China
- ProdList:142
- Advantage:58
-
Supplier:
Shanghai Daken Advanced Materials Co.,Ltd
- Tel:+86-371-+86-371-66670886
- Email:info@dakenam.com
- Country:China
- ProdList:19843
- Advantage:58
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
-
Supplier:
Fuxin Pharmaceutical
- Tel:+86-021-021-50872116<br/>+8613122107989
- Email:contact@fuxinpharm.com
- Country:China
- ProdList:10035
- Advantage:58
-
Supplier:
Guangzhou Yuheng Pharmaceutical Technology Co., Ltd
- Tel: +8613580539051
- Email:joe@yuhengpharm.com
- Country:CHINA
- ProdList:21142
- Advantage:58
-
Supplier:
Nantong Haigine Pharma Technology Co., Ltd
- Tel:0086-513-86266699
- Email:
- Country:CHINA
- ProdList:156
- Advantage:58
-
Supplier:
Hebei Bonster Technology Co.,Limited
- Tel: +8613315996897
- Email:bsterltd.wendy@gmail.com
- Country:China
- ProdList:792
- Advantage:58
-
Supplier:
Pharmahands (Taizhou) Co., Ltd.
- Tel:+86 523-86200360
- Email:
- Country:CHINA
- ProdList:358
- Advantage:58
-
Supplier:
CR Corporation Limited
- Tel: +8613062833949
- Email:fred.wen@crcorporation.cn
- Country:China
- ProdList:8647
- Advantage:58
917342-29-1, CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl esterRelated Search:
- Desmethyl Cariprazine Piperazine, 1-(2-chloroethyl)-4-(2,3-dichlorophenyl)- Urea, N,N'-bis[trans-4-[2-[4-(2,3-dichlorophenyl)-1-piperazinyl]ethyl]cyclohexyl]- Urea, N,N-dimethyl-N'-[trans-4-[2-[[(4-methylphenyl)sulfonyl]oxy]ethyl]cyclohexyl]- ethyl dimethylcarbamate 3,3-dimethyl-1-[(1r,4r)-4-(2-hydroxyethyl)cyclohexyl]urea Cyclohexaneethanol, 4-amino-, hydrochloride (1:1), trans- trans-4-Aminocyclohexanol Ethyl trans-2-(4-Aminocyclohexyl)acetate Hydrochloride Ethyl trans-2-[4-(Boc-aMino)cyclohexyl]acetate
- 917342-29-1
- 卡利拉嗪中间体
- 化学试剂
- 高端化学
- 盐酸卡利拉嗪中间体
- 反式-1-(BOC-氨基)-4-(2-羟乙基)环己烷/反式-4-BOC-氨基环己乙醇
- N-[(1S,4S)-4-(2-羟乙基)环己基]氨基甲酸叔丁酯
- ERT-BUTYL TRANS-4-(2-HYDROXYETHYL)-CYCLOHEXYLCARBAMATE
- 反式-1-(BOC-氨基)-4-(2-羟乙基)环己烷 卡利拉嗪中间体
- 叔-丁基反-4-(2-羟基乙基)环己基氨基甲酯
- (反式-4-(2-羟乙基)环己基)氨基甲酸叔丁酯
- 反式-4-(BOC-氨基)环己烷乙醇
- 反式-2-(4-BOC-氨基环己基)乙醇
- 反式-1-(BOC-氨基)-4-(2-羟乙基)环己烷
- [反式-4-(2-羟基乙基)环己基]氨基甲酸叔丁酯
- 反式-1-(BOC-氨基)-4-(2-羟乙基)环己烷 100G
- 917342-29-1
- tert-butyl N-[4-(2-hydroxyethyl)cyclohexyl]carbamate
- ert-Butyltrans-4-(2-hydroxyethyl)-cyclohexylcarbamate
- trans-4-(Boc-amino)cyclohexaneethanol
- trans 2-{1-[4-(N-(tert-butoxycarbonyl))-amino]-cyclohexyl}ethanol
- trans 2-{1-[4-(N-(tert-butoxycarbonyl))-amino]-cyclohexyl}et...
- trans-2-[4-[(tert-Butoxycarbonyl)amino]cyclohexyl]ethanol
- N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl esterCarbaMic acid
- trans-1-(Boc-aMino)-4-(2-hydroxyethyl)cyclohexane, 97%
- CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl ester
![CarbaMic acid, N-[trans-4-(2-hydroxyethyl)cyclohexyl]-, 1,1-diMethylethyl ester Structure](https://www.chemicalbook.com/CAS/GIF/917342-29-1.gif)