ChemicalBook > CAS DataBase List > Verapamil
Verapamil
Verapamil
- CAS No.52-53-9
- Chemical Name:Verapamil
- CBNumber:CB5376232
- Molecular Formula:C27H38N2O4
- Formula Weight:454.61
- MOL File:52-53-9.mol
Verapamil Property
- Melting point 25°C
- Boiling point 243-246 °C (1.3 Pa)
- Density 1.1267 (rough estimate)
- refractive index 1.5448
- storage temp. Keep in dark place,Inert atmosphere,Room temperature
- solubility DMSO: 100 mg/mL (219.97 mM)
- form Thick Oil
- pka 8.6(at 25℃)
- color Colourless
- CAS DataBase Reference 52-53-9(CAS DataBase Reference)
- EWG's Food Scores 1
- FDA UNII CJ0O37KU29
- NCI Drug Dictionary verapamil
- ATC code C08DA01,C08DA51
- NIST Chemistry Reference Verapamil(52-53-9)
- EPA Substance Registry System Verapamil (52-53-9)
Safety
- Hazardous Substances Data :52-53-9(Hazardous Substances Data)
- Toxicity :LD50 oral in rat: 163mg/kg
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H302-H315-H319-H335
- Precautionary statements P261-P280-P301+P312-P302+P352-P305+P351+P338
Verapamil Chemical Properties,Usage,Production
- Description Verapamil, the classic calcium antagonist, has a negative inotropic, anti-ischemic, and conduction-delaying effect on the heart , . Its antiarrhythmic effect is based primarily on a prolongation of impulse conduction time in theAVnode and a reduction of frequency of impulse generation in the SA node.
- Originator Isoptin,Knoll ,W. Germany ,1963
- Uses Verapamil is primarily used as an antiarrythmic for treating ventricular arrhythmias; however, currently it is being forced out gradually by adenosine.
- Uses Vasodilator (coronary).
- Uses Verapamil is used for preventing angina pectoris attacks, arterial hypertension, and treating and preventing supraventricular arrhythmia (paroxysmal supraventricular tachycardia, atrial fibrillation, atrial flutter, extrasystole).
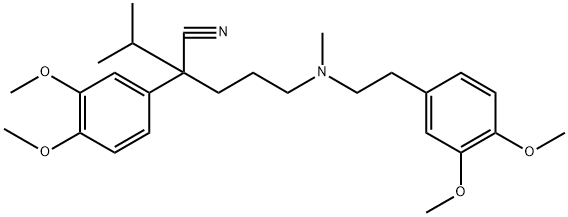
- Definition ChEBI: A tertiary amino compound that is 3,4-dimethoxyphenylethylamine in which the hydrogens attached to the nitrogen are replaced by a methyl group and a 4-cyano-4-(3,4-dimethoxyphenyl)-5-methylhexyl group.
-
Manufacturing Process
177.2 g (1 mol) of veratryl cyanide are dissolved in 1 liter of toluene in a
three-neck flask. 42.9 g (1.1 mols) of pulverized sodium amide are added.
The mixture is heated to boiling under reflux for one hour while stirring and
excluding moisture. A solution of the base (N-methyl-N-homoveratryl)-γ-
aminochloropropane, freshly prepared from 339.2 g (1.1 mols) of the
hydrochloride, in 1.2 liters of toluene is added drop by drop into this boiling
mixture within two hours while stirring vigorously. Heating and stirring are
continued for four more hours. After cooling, the reaction mixture is poured
into 3 liters of ice water while stirring, The mixture is acidified with 20%
hydrochloric acid. The acidified aqueous layer is separated, neutralized by the
addition of sodium hydroxide solution, and rendered alkaline by the addition
of concentrated potassium carbonate solution. The precipitated oily base is
taken up in benzene. On evaporating the solvent, 402 g of the crude base are
obtained in the form of a reddish-brown, viscous oil.
The crude base is dissolved in a mixture of 550 ml of isopropanol and 650 ml of ethyl acetate; Gaseous hydrogen chloride is introduced into the solution until it is of weakly acidic reaction. On allowing the mixture to stand at 0°C, 365 g of α-[(N-methyl-N-homoveratryl)-γ-amino-propyl]-3,4-dimethoxyphenyl acetonitrile hydrochloride precipitate as a slightly yellowish crystal powder of the melting point 136°C to 139°C (corr.). Yield: 81% of the theoretical yield. The pure, white hydrochloride melting at 140°C to 142°C (corr.) is obtained on recrystallizing the crude salt twice from isopropanol with the addition of decolorizing carbon. The salt is very soluble in water. The base prepared from the hydrochloride in the form of an almost colorless, very viscous oil boils at 233°C to 235°C/0.01 mm Hg; nD25= 1.5532. Dioxalate, melting point: 123°C to 125°C (corr.), on recrystallization from acetone and isopropanol.
61.9 g (0.15 mol) of α-[(N-methyl-N-homoveratryl)-γ-aminopropyl]-3,4- dimethoxyphenyl acetonitrile are dissolved in 300 ml of toluene. The solution is heated to boiling under reflux with 8.5 g (1.45 x 0.15 mols) of pulverized sodium amide for one hour while stirring. Thereafter, a solution of 31.4 g (1.7 x 0.15 mols) of isopropyl bromide in 50 ml of toluene is added drop by drop thereto within 90 minutes and the mixture is kept boiling for four more hours while stirring. The cooled reaction mixture is allowed to run into 1.5 liters of ice water and the mixture is acidified with 20% hydrochloric acid. The aqueous layer is separated and is rendered alkaline by the addition of a solution of potassium carbonate. The base is taken up in warm benzene. The solvent is evaporated and the residue is distilled in a vacuum. 62.6 g of α- isopropyl-α-[(N-methyl-N-homoveratryl)-γ-aminopropy]-3,4-dimethoxyphenyl acetonitrile are obtained in the form of a light yellow, very viscous oil. Boiling point: 232°C to 235°C/0.01 mm Hg; n D 25 = 1.5460. Yield: 91.8% of the theoretical yield. Hydrochloride: melting point: 139.5°C to 140.5°C (corr.), on recrystallization from a mixture of isopropanol and ethyl acetate. - Therapeutic Function Coronary vasodilator, Antiarrhythmic
-
General Description
Verapamil, 5-[. Hemodynamically, verapamil causesa change in the preload, afterload, contractility, heart rate,and coronary blood flow. The drug reduces systemic vascularresistance and mean blood pressure, with minor effectson cardiac output.
Verapamil is a synthetic compound possessing slightstructural similarity to papaverine. It can be separated intoits optically active isomers, of which the levorotatory enantiomeris the most potent. It is absorbed rapidly after oraladministration. The drug is metabolized quickly and, as aresult, has low bioavailability. The liver is the main siteof first-pass metabolism, forming several products. Thepreferential metabolic step involves N-dealkylation, followedby O-demethylation, and subsequent conjugation ofthe product before elimination. The metabolites have no significantbiological activity. Verapamil has an eliminationhalf-life of approximately 5 hours. - Mechanism of action Verapamil is used as an antiarrythmic drug in treating supraventricular arrythmia such as paroxysmal atrial tachycardia, and for controlling atrial fibrillation. By blocking entrance of Ca2+ in the cell, verapamil exhibits a negative inotropic effect, and therefore it cannot be combined with β-adrenoblockers or cynidine since that would lead to an increased inotropic effect.
-
Clinical Use
Verapamil (Isoptin, Covera), in addition to its use as an
antiarrhythmic agent, has been employed extensively in
the management of variant (Prinzmetal’s) angina and
effort-induced angina pectoris. It selectively inhibits the voltage-gated calcium
channel that is vital for action potential genesis in slowresponse
myocytes, such as those found in the sinoatrial
and A-V nodes.
Verapamil is useful for slowing the ventricular response to atrial tachyarrhythmias, such as atrial flutter and fibrillation. Verapamil is also effective in arrhythmias supported by enhanced automaticity, such as ectopic atrial tachycardia and idiopathic left ventricular tachycardia. - Side effects Orally administered verapamil is well tolerated by most patients. Most complaints are of constipation and gastric discomfort. Other complaints include vertigo, headache, nervousness, and pruritus.
-
Synthesis
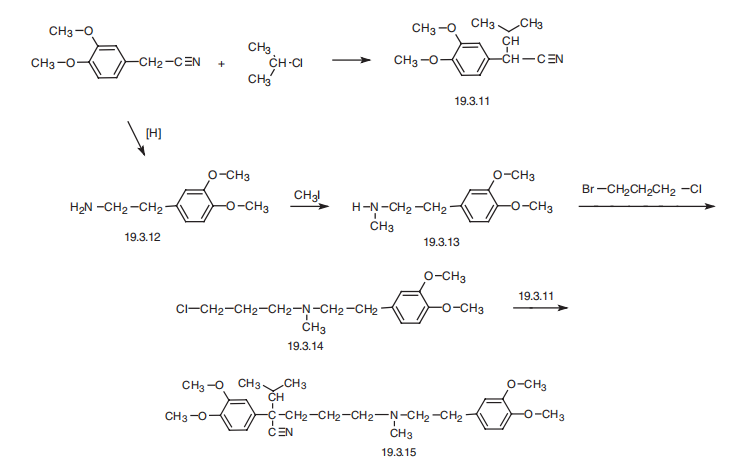
Verapamil, 5-[(3,4-dimethoxyphenethyl)methylamino]-2-(3,4-dimethoxyphenyl)
isopropylvaleronitrile (19.3.15), is synthesized by a scheme using 3,4-
dimethoxyphenylacetonitrile as the initial substance. The synthesis of the final product
(19.3.15) is accomplished by alkylating 2-(3.4-dimethoxyphenyl)-3-methylbutyronitrile
(19.3.11) with N-[2-(3,4-dimethoxyphenyl)-ethyl]-N-3-(chloropropyl)-N-methylamine
(19.3.14). The initial 2-(3.4-dimethoxyphenyl)-3-methylbutyronitrile (19.3.11) is synthesized
by alkylating 3,4-dimethoxyphenylacetonitrile with isopropyl chloride in the
presence of sodium amide. The alkylating agent, N-[2-(3,4-dimethoxyphenyl)-ethyl]-N-3-
(chloropropyl)-N-methylamine (19.3.14), is also synthesized from 3,4-dimethoxyphenylacetonitrile
followed by reduction into 3,4-dimethoxyphenylethylamine (19.3.12), with
subsequent methylation into N-methyl-N-3,4-dimethoxyphenylethylamine (19.3.13).
Next, the resulting N-[2-(3,4-dimethoxyphenyl)-ethyl] -N-methylamine (19.3.12) is
alkylated by 1-chloro-3-bromopropane into the desired N-[2-(3,4-dimethoxyphenyl)-
ethyl]-N-3-(chloropropyl)-N-methylamine (19.3.14), which is alkylated by 2-(3.4-
dimethoxyphenyl)-3-methylbutyronitrile (19.3.11) to give the final product, verapamil(19.3.15).
- Precautions Verapamil must be used with extreme caution or not at all in patients who are receiving -adrenoceptor blocking agents. Normally, the negative chronotropic effect of verapamil will in part be overcome by an increase in reflex sympathetic tone. The latter is be prevented by simultaneous administration of a β-adrenoceptor blocking agent, which exaggerates the depressant effects of verapamil on heart rate, A-V node conduction, and myocardial contractility. The use of verapamil in children less than 1 year of age is controversial.
Verapamil Preparation Products And Raw materials
Raw materials
Preparation Products
Global(145)Suppliers
-
Supplier:
SHANDONG ZHI SHANG CHEMICAL CO.LTD
- Tel: +86 18953170293
- Email:sales@sdzschem.com
- Country:China
- ProdList:2930
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
-
Supplier:
Casorganics US Corp
- Tel:+17326109938
- Email:sales@casorganics.com
- Country:CHINA
- ProdList:174
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Hefei TNJ Chemical Industry Co.,Ltd.
- Tel:+86-0551-65418671<br/>+8618949823763
- Email:sales@tnjchem.com
- Country:China
- ProdList:34563
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-029-89586680<br/>+86-18192503167
- Email:1026@dideu.com
- Country:China
- ProdList:7859
- Advantage:58
-
Supplier:
Finetech Industry Limited
- Tel:+86-27-8746-5837<br/>+8619945049750
- Email:info@finetechnology-ind.com
- Country:China
- ProdList:9642
- Advantage:58
-
Supplier:
LUYUNJIA CHEMISTRY XIAMEN LIMITED
- Tel:+86-592-5360779<br/>+86-13055435203
- Email:
- Country:China
- ProdList:5988
- Advantage:58
-
Supplier:
LEAPCHEM CO., LTD.
- Tel:+86-852-30606658
- Email:market18@leapchem.com
- Country:China
- ProdList:43340
- Advantage:58
52-53-9, VerapamilRelated Search:
- Methyl acetate Methyl acrylate 3-Chloropropyltrimethoxysilane Diphenyldimethoxysilane Dimethyldimethoxysilane 1,1-Dimethoxyethane Methylparaben Bensulfuron methyl Kresoxim-methyl METHYL THIOPHENE-2-CARBOXYLATE N,N-Diisopropylethylamine Trimethoxypropylsilane 4-Methoxyphenylacetone Thiophanate-methyl Methyl Methyl bromide (+)-VERAPAMIL, METHOXY-, HYDROCHLORIDE,R(+)-VERAPAMIL HYDROCHLORIDE,Verapamil HCI Parathion-methyl
- Verapamil
- 抑制剂
- FDA批准的配体
- 原料药
- C27H37N2O4
- C27H38N2O4
- 维拉帕米,10 MM DMSO 溶液
- 维拉帕米检测标准品
- 维拉帕米(异博定)
- 维拉帕米相关杂质
- 维拉帕米杂质P
- 维拉帕米杂质N
- 异博定
- 戊脉胺
- 维拉帕米
- 5-((3,4-二甲氧基苯乙基)甲基氨基)-2-(3,4-二甲氧基苯基)-2-异丙基戊腈
- 52-53-9
- Verapamil, 10 mM in DMSO
- Verapamil in methanol
- Vasolan
- Dilacoran
- VerapamilQ: What is Verapamil Q: What is the CAS Number of Verapamil Q: What is the storage condition of Verapamil Q: What are the applications of Verapamil
- Verapamil USP/EP/BP
- verapaiTiil
- Benzeneacetonitrile, α-[3-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]propyl]-3,4-dimethoxy-α-(1-methylethyl)-
- (2S)-2-(3,4-dimethoxyphenyl)-5-[2-(3,4-dimethoxyphenyl)ethyl-methylamino]-2-propan-2-ylpentanenitrile
- CP 16533-1 (Verapamil)
- VerapamilC27H38N204
- Verapamil (base and/or unspecified salts)
- VERAPAMIL
- (+/-) VERAPAMIL
- valeronitrile,5-((3,4-dimethoxyphenethyl)methylamino)-2-(3,4-dimethoxyphenyl)
- Valeronitrile, 5-((3,4-dimethoxyphenethyl)methylamino)-2-(3,4-dimethoxyphenyl)-2-isopropyl-
- oxyphenylacetonitrile
- ha-(1-methylethyl)benzeneacetonitrile
- eronitrile
- D-365
- CP-16533-1
- benzeneacetonitrile,alpha-(3-((2-(3,4-dimethoxyphenyl)ethyl)methylamino)propy
- Benzeneacetonitrile, alpha-[3-[[2-(3,4-dimethoxyphenyl)ethyl]methylamino]propyl]-3,4-dimethoxy-alpha-(1-methylethyl)-
- alpha-isopropyl-alpha-[(n-methyl-n-homoveratryl)-gamma-aminopropyl]-3,4-dimeth
- alpha-[3-[[2-(3,4-dimethoxyphenyl)ethyl]-methylamino]propyl]-3,4-dimethoxy-alp
- alpha-((N-Methyl-N-homoveratryl)-gamma-aminopropyl)-3,4-dimethoxyphenylacetonitrile
- alpha-((n-methyl-n-homoveratryl)-gamma-aminopropyl)-3,4-dimethoxyphenylaceto
- aleronitrile
- 5-((3,4-Dimethoxyphenethyl)methylamino)-2-(3,4-dimethoxyphenyl)-2-isopropylvaleronitrile
- 5-((3,4-dimethoxyphenethyl)methylamino)-2-(3,4-dimethoxyphenyl)-2-isopropylval
- verapamile
- Lekoptin
- (2R)-2-(3,4-diMethoxyphenyl)-5-{[2-(3,4-diMethoxyphenyl)ethyl](Methyl)aMino}-2-(propan-2-yl)pentanenitrile
- alpha-[3-[[2-(3,4-Dimethoxyphenyl)ethyl]methylamino]propyl]-3,4-dimethoxy-alpha-(1-methylethyl)benzeneacetonitrile
- 5-((3,4-Dimethoxyphenethyl)(methyl)amino)-2-(3,4-dimethoxyphenyl)-2-isopropylpentanenitrile
- 2-(3,4-Dimethoxyphenyl)-5-[2-(3,4-dimethoxyphenyl)ethyl-methylamino]-2-propan-2-ylpentanenitrile
- (2R)-2-(3,4-Dimethoxyphenyl)-5-{[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino}-2-isopropylpentanenitrile
- 2-(3,4-Dimethoxyphenyl)-5-[[2-(3,4-dimethoxyphenyl)ethyl](methyl)amino]-2-isopropylpentanenitrile
- VPL
- Valeronitrile, 5-[(3,4-dimethoxyphenethyl)methylamino]-2-(3,4-dimethoxyphenyl)-2-isopropyl- (7CI, 8CI)
- R,S-Verapamil