ChemicalBook > CAS DataBase List > Regorafenib monohydrate
Regorafenib monohydrate
Regorafenib monohydrate
- CAS No.1019206-88-2
- Chemical Name:Regorafenib monohydrate
- CBNumber:CB52641189
- Molecular Formula:C21H17ClF4N4O4
- Formula Weight:500.83
- MOL File:1019206-88-2.mol
Regorafenib monohydrate Property
- storage temp. 2-8°C
- solubility ≥25.05 mg/mL in DMSO; insoluble in H2O
- form solid
- color Light yellow to orange
- InChIKey ZOPOQLDXFHBOIH-UHFFFAOYSA-N
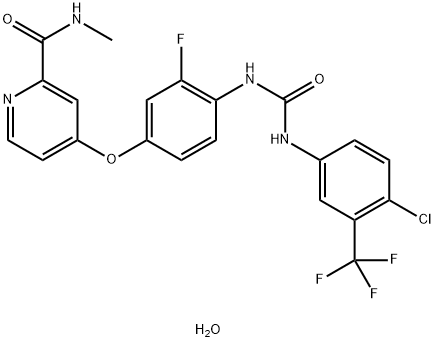
- SMILES O(C1C=CC(NC(=O)NC2C=CC(Cl)=C(C(F)(F)F)C=2)=C(F)C=1)C1=CC=NC(C(=O)NC)=C1.O
- FDA UNII MGN125FS9D
- UNSPSC Code 41116107
- NACRES NA.24
-
Symbol(GHS)


- Signal wordWarning
- Hazard statements H361fd-H373-H410
- Precautionary statements P201-P273-P308+P313
Regorafenib monohydrate Chemical Properties,Usage,Production
- Description Regorafenib monohydrate (BAY 73-4506) is an orally active and potent multi-targeted receptor tyrosine kinase inhibitor with potent anti-tumour and anti-angiogenic activity. It is approved for the treatment of metastatic colorectal cancer, advanced gastrointestinal mesenchymal stromal tumours and hepatocellular carcinoma.
- Uses 4-[4-[[4-Chloro-3-(trifluoromethyl)phenyl]carbamoylamino]-3-fluorophenoxy]-N-methylpyridine-2-carboxamide Hydrate can be used as an anti-tumor drug.
- Definition ChEBI: Regorafenib hydrate is a hydrate that is the monohydrate form of anhydrous regorafenib. Used for for the treatment of metastatic colorectal cancer in patients who have previously received chemotherapy, anti-EGFR or anti-VEGF therapy. It has a role as an antineoplastic agent, a tyrosine kinase inhibitor and a hepatotoxic agent. It contains a regorafenib.
- Biological Activity regorafenib monohydrate is a multitargetedinhibitor of tyrosine kinase with ic50 values of 13nm, 4.2nm, 46nm, 2.5nm, 28nm, 19nm, 202nm, 22nm, 7nm, 1.5nm and 311nm, respectively for vegfr-1, mvegfr-2, mvegfr-3, raf-1, braf wt, brafv600e, fgfr-1, pdgfr-β, c-kit, retand tie2 [1].regorafenib is a multikinase inhibitor of both intracellular and membrane-bound rtks. it shows potent inhibition of angiogenic and stromal rtks like vegf receptors-1-3, pdgfr-β and fgf receptor-1 with ic50 values ranging from4.2 to 311nm in biochemical assays. it also inhibits oncogenic rtks, such as ret and c-kit, with ic50 values ranging from 1.5 to 28nm in cellular assays [1].regorafenib is reported to have anti-tumor efficacy to various tumors including breast, pancreas, thyroid, melanoma, gist, and crc with a mean ic50 value less than 1μm. these inhibition effects of tumor growth are also found in mouse xenograft models after the treatment of regorafenib at dose ranging from 10 to 100 mg/kg [1].
- Mechanism of action The mechanism of anticancer action of Regorafenib monohydrate is similar to that of Regorafenib, an oral multikinase inhibitor that blocks tyrosine kinases that are active in angiogenesis, cancer development and growth, and maintenance of the tumour microenvironment. It is superior to sorafenib in blocking both vascular endothelial growth factor receptor and TIE2, a molecule with an important role in angiogenesis.
- Clinical Use Regorafenib was approved by the U.S. Food and Drug Administration (FDA) in September 2012 for the treatment of metastatic colorectal cancer in patients who have previously undergone fluoropyrimidine-, oxaliplatin-, and irinotecan-based therapies. The FDA expanded the approved use of the drug to include patients with advanced gastrointestinal stromal tumors (GIST) that cannot be surgically removed and no longer respond to imatinib and sunitinib, two other drugs approved for treatment of GIST. Regorafenib, marketed under the trade name Stivarga®, was discovered and developed by Bayer Pharmaceuticals and marketed jointly with Onyx Pharmaceuticals. The active metabolites of the drug inhibit multiple targets within a variety of kinase families including those in the RET, VEGF, FGFR, PTK, and Abl pathways.
- Side effects Common side effects of regorafenib monohydrate include hand-foot skin reaction, diarrhea, nausea, vomiting, decreased appetite, fatigue, hypertension, and fever.
-
Synthesis
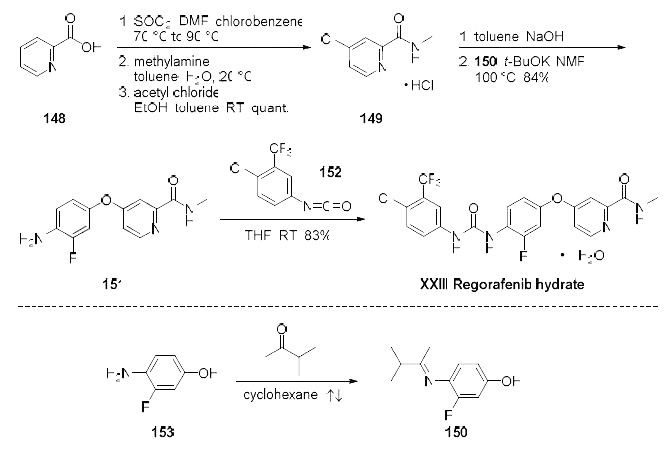
Among several published synthesis, the most likely process scale synthesis will be highlighted
from the two published syntheses, and this is described in the scheme. Commercially available
picolinic acid (148) was heated with thionyl chloride to provide the crude intermediate 4-chloro-2-
pyridyl acid chloride which was subsequently reacted with aqueous methyl amine in toluene to give 4-
chloro-2-methylcarboxamide as its hydrochloride salt 149 in quantitative yield after treatment with
acetyl chloride in toluene and ethanol. The hydrochloride salt was free based with sodium hydroxide
and then immediately reacted with imine 150 (formed upon exposure to 4-amino-3-fluorophenol (153)
in refluxing 3-methyl 2-butanone) in base to provide diaryl ether 151 in 84% yield. Reaction of amine
151 with the commercially available isocyanate 152 ultimately delivered regorafenib hydrate (XXIII) in
83% yield.
- References [1] crona dj, keisler md, walko cm.regorafenib: a novel multitargeted tyrosine kinase inhibitor for colorectal cancer and gastrointestinal stromal tumors.ann pharmacother. 2013 dec;47(12):1685-96.
Regorafenib monohydrate Preparation Products And Raw materials
Raw materials
Preparation Products
Global(256)Suppliers
-
Supplier:
Yangzhou Qinyuan Pharmatech Co.,ltd
- Tel: +86-18752526868
- Email:jennysun@yzqyyykj.com
- Country:China
- ProdList:81
- Advantage:58
-
Supplier:
BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD.
- Tel:+86-18600796368<br/>+86-18600796368
- Email:sales@sjar-tech.com
- Country:China
- ProdList:485
- Advantage:58
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5854
- Advantage:58
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
-
Supplier:
Beijing Cooperate Pharmaceutical Co.,Ltd
- Tel:010-60279497
- Email:sales01@cooperate-pharm.com
- Country:CHINA
- ProdList:1803
- Advantage:55
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Nanjing ChemLin Chemical Industry Co., Ltd.
- Tel:025-83697070
- Email:product@chemlin.com.cn
- Country:CHINA
- ProdList:3009
- Advantage:60
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
Anqing Chico Pharmaceutical Co., Ltd.
- Tel:15380796838
- Email:chloewu@chicopharm.cn
- Country:CHINA
- ProdList:340
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
1019206-88-2, Regorafenib monohydrateRelated Search:
- Alectinib Hydrochloride PLX4032 Nintedanib Ethanesulfonate Salt Olaparib 4-Amino-3-fluorophenol 4-(4-AMINO-3-FLUOROPHENOXY)-N-METHYLPICOLINAMIDE 4-Chloro-alpha,alpha,alpha-trifluoro-m-toluidine Regorafenib Crizotinib Afatinib (BIBW 2992) PLERIXAFOR Regorafenib (Hydrochloride) SOLITHROMYCIN Afatinib dimaleate Dacomitinib (PF299804) Cabozantinib Malate 918504-65-1 1110813-31-4
- API
- 药靶配体
- 化学试剂
- 黄金产品
- 日用化学品
- 科研出口类
- 杂质对照品
- 原料
- 细胞生物学试剂
- 优势产品
- 抑制剂
- 医药原料
- 抗癌类
- 原料药
- C21H17ClF4N4O4
- C21H15ClF4N4O3H2O
- 瑞格非尼一水合物,10 MM DMSO 溶液
- 雷戈拉非尼(BAY-734506)一水合物
- 瑞戈菲尼水合物
- 瑞戈非尼-水物
- 瑞戈非尼一水
- 4-[4-[[[[[4-氯-3-(三氟甲基)苯基]氨基]羰基]氨基]-3-氟苯氧基]-N-甲基-2-吡啶甲酰胺水合物
- 阿利色替
- 瑞戈菲尼杂质1
- 4-(4-(3-(4-氯-3-(三氟甲基)苯基)脲基)-3-氟苯氧基)-N-甲基吡啶酰胺水合物
- 多靶点抑制剂(REGORAFENIB MONOHYDRATE)
- 瑞格菲屏融
- 瑞戈非尼一水物
- 瑞格菲尼水合物/4- [4 - [[[[4-氯-3-(三氟甲基)苯基]氨基]羰基]氨基] -3-氟苯氧基] -N-甲基-2-吡啶甲酰胺水合物
- 瑞戈非尼一水合物
- 瑞戈非尼水合物
- 瑞格非尼一水合物
- 瑞格菲尼一水物
- 瑞格菲尼一水合物
- 瑞格菲尼水合物
- 瑞格非尼(水合物)
- 1019206-88-2
- Regorafenib monohydrate, 10 mM in DMSO
- Regfini hydrate
- 4-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide hydrate , Regorafenib monohydrate
- Regorafenib (200 mg)
- Regfenib monohydrate
- Refined Sunflower Oil
- 4-[4-[[[[4-Chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]-3-fluorophenoxy]-N-methyl-2-pyridi
- 4-[4-[[[[4-Chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]-3-fluorophenoxy]-N-methyl-2-pyridinecarboxamide hydrate USP/EP/BP
- Regorafenibhydrate USP/EP/BP
- 4-(4-(3-(4-chloro-3-(trifluoromethyl)phenyl)ureido)-3-fluorophenoxy)-N-methylpicolinamide hydrate
- Regorafenib H2O
- Regorafenib monohydrate (BAY 73-4506)
- 4-[4-[[4-chloro-3-(trifluoromethyl)phenyl]carbamoylamino]-3-fluorophenoxy]-N-methylpyridine-2-carboxamide,hydrate
- 4-[4-({[4-Chloro-3-(trifluoromethyl)phenyl]carbamoyl}amino)-3-fluorophenoxy]-N-methyl-2-pyridinecarboxamide hydrate (1:1)
- Regorafenib monohydrate 4-[4-[[[[4-Chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]-3-fluorophenoxy]-N-methyl-2-pyridinecarboxamide hydrate
- BAY 73-4506 Monohydrate
- Regorafenib hydrate
- Regorafenib (BAY 73-4506)Monohydrate
- Regorafenib monohydrate
- 4-[4-[[[[4-Chloro-3-(trifluoromethyl)phenyl]amino]carbonyl]amino]-3-fluorophenoxy]-N-methyl-2-pyridinecarboxamide hydrate