ChemicalBook > CAS DataBase List > Isoleucine
Isoleucine
Isoleucine
- CAS No.7004-09-3
- Chemical Name:Isoleucine
- CBNumber:CB52130861
- Molecular Formula:C6H13NO2
- Formula Weight:131.17
- MOL File:7004-09-3.mol
Isoleucine Property
- Melting point >166°C (dec.)
- storage temp. Refrigerator
- solubility Aqueous Acid (Slightly), DMSO (Slightly, Sonicated)
- form Solid
- pka 2.32(at 25℃)
- color White to Off-White
- FDA 21 CFR 582.5381; 310.545
- EWG's Food Scores 1
- FDA UNII 04Y7590D77
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
Isoleucine Chemical Properties,Usage,Production
-
Description
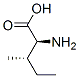
Isoleucine ( abbreviated as Ile or I ) is an α - amino acid with the chemical formula HO2CCH(NH2)CH(CH3)CH2CH3. It is an essential amino acid, which means that humans cannot synthesize it, so it must be ingested. Its codons are AUU, AUC and AUA.
With a hydrocarbon side chain, isoleucine is classified as a hydrophobic amino acid. Together with threonine, isoleucine is one of two common amino acids that have a chiral side chain. Four stereoisomers of isoleucine are possible, including two possible diastereomers of L-isoleucine. However, isoleucine present in nature exists in one enantiomeric form, (2S,3S)-2-amino-3-methylpentanoic acid. - Chemical Properties Crystals. Slightly soluble in water; nearly insoluble in alcohol; insoluble in ether.
- Occurrence Even though this amino acid is not produced in animals, it is stored in high quantities. Foods that have high amounts of isoleucine include eggs, soy protein, seaweed, turkey, chicken, lamb, cheese, and fish.
- Uses Amino acid.
- Definition An essential amino acid, found naturally in the L(+) form.
-
Biosynthesis
As an essential nutrient, it is not synthesized in the body, hence it must be ingested, usually as a component of proteins. In plants and microorganisms, it is synthesized via several steps, starting from pyruvic acid and alpha - keto glutarate. Enzymes involved in this biosynthesis include :
Aceto lactate synthase (also known as aceto hydroxy acid synthase)
Aceto hydroxy acid iso meroreductase
Dihydroxy acid dehydratase
Valine amino transferase. -
Synthesis
Isoleucine can be synthesized in a multistep procedure starting from 2-bromobutane and diethylmalonate.Synthetic isoleucine was originally reported in 1905.
German chemist Felix Ehrlich discovered isoleucine in hemoglobin in 1903. - Metabolism Isoleucine is both a glucogenic and a ketogenic amino acid. After transamination with alpha-ketoglutarate the carbon skeleton can be converted into either Succinyl CoA, and fed into the TCA cycle for oxidation or converted into oxaloacetate for gluconeogenesis (hence glucogenic). It can also be converted into Acetyl CoA and fed into the TCA cycle by condensing with oxaloacetate to form citrate. In mammals Acetyl CoA cannot be converted back to carbohydrate but can be used in the synthesis of ketone bodies or fatty acids, hence ketogenic.
Isoleucine Preparation Products And Raw materials
Raw materials
Preparation Products
Global(12)Suppliers
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
- Supplier: Nanjing Shizhou Biology Technology Co.,Ltd
- Tel: 13675144456
- Email:sean.lv@synzest.com
- Country:China
- ProdList:10784
- Advantage:58
- Isoleucine
- Isoleucine
- 亮氨酸EP杂质A
- 7004-09-3
- ISOLEUCINE