ChemicalBook > CAS DataBase List > Atazanavir sulfate
Atazanavir sulfate
Atazanavir sulfate
- CAS No.229975-97-7
- Chemical Name:Atazanavir sulfate
- CBNumber:CB3547456
- Molecular Formula:C38H54N6O11S
- Formula Weight:802.94
- MOL File:229975-97-7.mol
Atazanavir sulfate Property
- Melting point 195.0°, or acetone; mp 198-199° (dec)
- alpha D22 -46.1° (c = 1 in 1:1 CH3OH/H2O, pH = 2.6)
- storage temp. under inert gas (nitrogen or Argon) at 2-8°C
- solubility ≥28.7 mg/mL in DMSO with gentle warming; insoluble in H2O; ≥4.05 mg/mL in EtOH with gentle warming and ultrasonic
- form Powder
- color White to light yellow
- optical activity [α]/D -40 to -50°, c =1 in methanol: water (1:1)
- Water Solubility H2O: 2mg/mL, clear
- FDA UNII 4MT4VIE29P
- UNSPSC Code 41116107
- NACRES NA.77
Safety
- Safety Statements :24/25
- HS Code :29333990
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H372-H318
- Precautionary statements P280-P305+P351+P338-P310-P260-P264-P270-P314-P501
Atazanavir sulfate Chemical Properties,Usage,Production
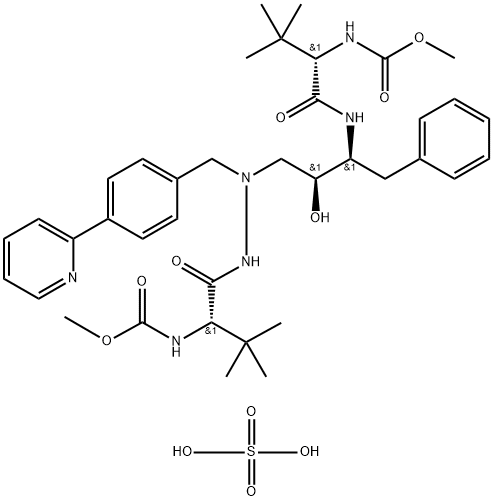
- Description Atazanavir (BMS-232632, III), an azapeptide HIV protease inhibitor, has been developed and launched by Bristol-Myers Squibb (BMS), under worldwide license from Novartis, for the treatment of HIV infection. Atazanavir was launched in the US as Reyataz™ in July 2003.
- Chemical Properties Off-White Solid
- Uses Atazanavir is a HIV protease inhibitor with Ki of 2.66 nM
- Uses Atazanavir Sulfate is an intermediate of Atazanavir(A790051) which is a novel azapeptide HIV protease Inhbitor. Antiviral.
- Uses Atazanavir is a novel azapeptide HIV protease inhibitor (PI). Antiviral.
- brand name Reyataz (Bristol-Myers Squibb).
-
Synthesis
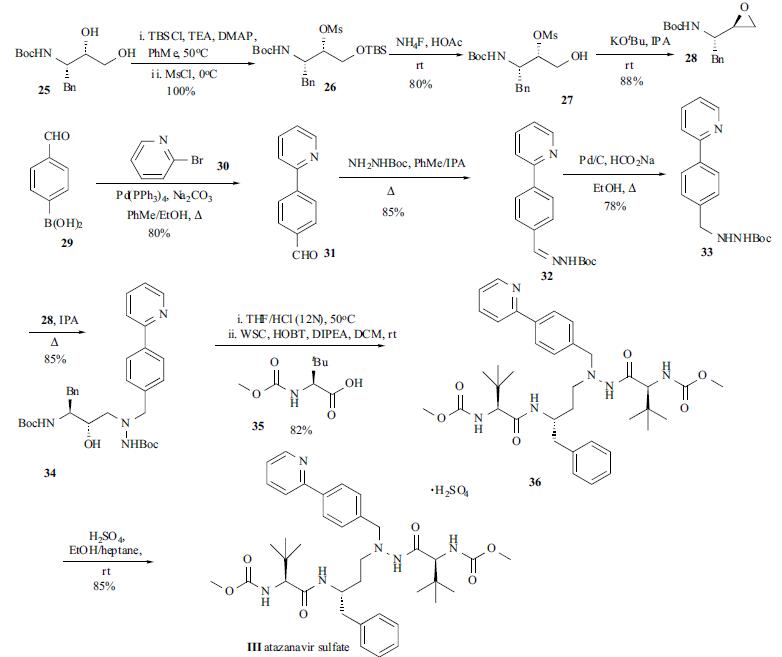
The
synthesis of atazanavir (III) appeared in several reports. The synthetic route depicted in the scheme was one of
the best routes which was suitable for large scale production. The commercially available chiral diol 25 was
converted to its silyl mesylate 26 in one pot via selective
silylation and subsequent mesylation. This oily intermediate
26 was carried into the next step without further purification.
The desilylation of 26 was achieved by using inexpensive
ammonium fluoride. The resulting solid product 27 was
readily isolated and further purified through recrystallization
from IPA/H2O in 80% yield. The epoxide formation from
27 was affected by KOtBu in THF/IPA to provide enantiomerically pure epoxide 28 in 88% yield. Suzuki
coupling of boronic acid 29 with bromopyridine (30)
provided pyridyl benzaldehyde 31 in 80% yield after
crystallization. The subsequent condensation of aldehyde 31
with t-butylcarbamate was carried out by refluxing in
toluene/IPA and Shiff base 32 was collected by filtration
upon cooling. Reduction of hydrazone 32 to hydrazine 33
was accomplished by employing a catalytic phase-transfer
hydrogenation protocol (Pd/C, HCOONa) to furnish
hydrazine 33 in 78% yield after crystallization. Coupling of
the hydrazinocarbamate 33 with epoxide 28 was performed
in refluxing IPA, followed by the addition of water to
precipitate the crude product. Subsequent recrystallization
from MeCN/H2O furnished 34 in 85% yield. Treatment of
34 with concentrated HCl in THF at 50oC removed the two
Boc groups in 34 to give the product as an oil, which was
then dissolved in a mixture of DCM/DIPEA and slowly
transferred into a premixed solution of N-methoxycarbonyl-
L-tert-leucine (35), HOBT, and WSC in DCM. After
removal of the solvent the crude product was crystallized
from IPA/EtOH to furnish the freebase 36 in 82% yield. The
sulfate III was obtained by stirring the free base 36 with
concentrated H2SO4 in EtOH at ambient temperature. Direct
crystallization by addition of n-heptane provided the sulfate
salt III as an easily filterable solid in 85% yield.
Atazanavir sulfate Preparation Products And Raw materials
Raw materials
Preparation Products
Global(303)Suppliers
-
Supplier:
Hangzhou Hyper Chemicals Limited
- Tel:+86-0086-57187702781<br/>+8613675893055
- Email:info@hyper-chem.com
- Country:China
- ProdList:295
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29858
- Advantage:58
-
Supplier:
Biochempartner
- Tel:0086-13720134139
- Email:candy@biochempartner.com
- Country:CHINA
- ProdList:965
- Advantage:58
-
Supplier:
HaBo Hong Kong Co., Limited.
- Tel:+86-25-18512596065
- Email:info@habotech.com
- Country:CHINA
- ProdList:933
- Advantage:58
-
Supplier:
Shandong chuangyingchemical Co., Ltd.
- Tel:18853181302
- Email:sale@chuangyingchem.com
- Country:CHINA
- ProdList:5906
- Advantage:58
-
Supplier:
Tianjin Xinshengjiahe Science & Technology Development Co,.Ltd
- Tel:+86-86-22-87899925<br/>+86-8618522618860
- Email:18522618860@163.com
- Country:China
- ProdList:694
- Advantage:58
-
Supplier:
TargetMol Chemicals Inc.
- Tel:+1-781-999-5354<br/>+1-00000000000
- Email:marketing@targetmol.com
- Country:United States
- ProdList:32159
- Advantage:58
-
Supplier:
Hubei Ipure Biology Co., Ltd
- Tel: +8613367258412
- Email:ada@ipurechemical.com
- Country:China
- ProdList:10244
- Advantage:58
229975-97-7, Atazanavir sulfateRelated Search:
- Kresoxim-methyl Sodium sulfate Dimethyl sulfate Methylparaben D-Glucosamine sulfate Bensulfuron methyl Aluminum sulfate Methyl acrylate CHLOROPHOSPHONAZO III Ammonium sulfate Magnesium sulfate heptahydrate Methyl acetate Zinc sulfate heptahydrate Atazanavir Thiophanate-methyl sulfate Methyl Methyl bromide
- 229975-97-7
- BMS-232632-05, Reyataz
- peptides
- Inhibitors
- Antiviral Agents
- Atazanavir
- Inhibitor
- 科研原料
- 生化试剂
- 药靶配体
- 抑制剂
- 化学试剂
- 化学原料
- 化工中间体
- 无机盐
- 医药原料
- 医用原料
- 医药原料
- 原料
- 医用原料
- 原料药API
- 蛋白酶
- 小分子抑制剂
- 抗生素
- 小分子抑制剂,天然产物
- 原料药
- 抗病毒类
- C38H52N6O7H2SO4
- 阿扎那韦杂质32
- 硫酸阿扎那韦,10 MM DMSO 溶液
- ((5S,10S,11S,14S)-11-苄基-5-(叔丁基)-10-羟基-15,15-二甲基-3,6,13-三氧代-8-(4-(吡啶-2-基)苄基)-2-氧杂-4,7,8,12-四氮杂十六烷-14-基)氨基甲酸甲酯 硫酸盐
- 阿扎那韦硫酸盐, HIV PROTEASE抑制剂
- 阿扎那韦硫酸盐
- 硫酸阿扎那韦
- 阿扎那韦硫酸盐 REYATAZ
- 阿扎那韦硫酸盐(标准品)
- 阿扎那韦(硫酸盐)(ATAZANAVIR)
- 229975-97-7
- ATAZANAVIR SULFATE USP (NON-STERILE DRUG SUBSTANCE)
- ATAZANAVIR SULPHATE [DRUG SUBSTANCE (NON-STERILE)]
- Atazanavir sulfate, 10 mM in DMSO
- Azanavir sulfate
- Atazanavir Sulfate (1044334)
- Atazanavir sulfateQ: What is Atazanavir sulfate Q: What is the CAS Number of Atazanavir sulfate Q: What is the storage condition of Atazanavir sulfate Q: What are the applications of Atazanavir sulfate
- Atazanavir Sulfate (Latazanavir, Zrivada, Reyataz, BMS232632)
- Atazanavir sulfate USP/EP/BP
- Dimethyl (3S,8S,9S,12S)-9-benzyl-3,12,di-tert-butyl-8-hydroxy-4,11-dioxo-6-(p-2-pyridylbenzyl)-2,5,6,10,13-pentaazatetradecanedioate sulfate
- ((5S,6S)-5-hydroxy-1,8-dioxo-6-(phenyl methyl)-3-[[4-(2- pyridinyl)phenyl]methyl] -2,3,7 triaza octane dioic acid tertiary butyl ester)
- Atazanavir sulfate CRS
- ATAZANAVIR SULFATE (BMS-232632 SULFATE)
- Methyl {(5S,10S,11S,14S)-11-benzyl-10-hydroxy-15,15-dimethyl-5-(2-methyl-2-propanyl)-3,6,13-trioxo-8-[4-(2-pyridinyl)benzyl]-2-oxa-4,7,8,12-tetraazahexadecan-14-yl}carbamate
- Atazanavir (BMS-232632) sulfate
- Atazanavir sulfate, 99%, HIV protease inhibitors
- BMS-232632 sulfate
- BMS232632 sulfate
- BMS 232632 SULFATE;BMS-232632 SULFATE;BMS232632 SULFATE
- BMS 232632 sulfate
- N-[(2S)-1-[2-[(2S,3S)-2-hydroxy-3-[[(2S)-2-(methoxycarbonylamino)-3,3-dimethyl-1-oxobutyl]amino]-4-phenylbutyl]-2-[(4-phenylphenyl)methyl]hydrazinyl]-3,3-dimethyl-1-oxobutan-2-yl]carbamic acid methyl