ChemicalBook > CAS DataBase List > Cefuroxime Sodium EP Impurity G
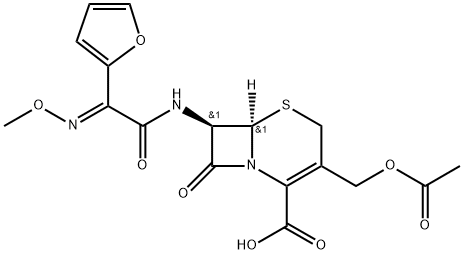
Cefuroxime Sodium EP Impurity G
Cefuroxime Sodium EP Impurity G
- CAS No.97232-98-9
- Chemical Name:Cefuroxime Sodium EP Impurity G
- CBNumber:CB33155005
- Molecular Formula:C17H17N3O8S
- Formula Weight:423.4
- MOL File:97232-98-9.mol
Cefuroxime Sodium EP Impurity G Property
- Density 1.63±0.1 g/cm3(Predicted)
- pka 2.66±0.50(Predicted)
- FDA UNII 9FA4SWZ5ZT
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
Cefuroxime Sodium EP Impurity G Preparation Products And Raw materials
Raw materials
Preparation Products
Global(51)Suppliers
-
Supplier:
Standardpharm Co. Ltd.
- Tel:86-714-3992388
- Email:overseasales1@yongstandards.com
- Country:United States
- ProdList:14332
- Advantage:58
-
Supplier:
Career Henan Chemica Co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:laboratory@coreychem.com
- Country:China
- ProdList:30231
- Advantage:58
-
Supplier:
ANHUI WITOP BIOTECH CO., LTD
- Tel: +8615255079626
- Email:eric@witopchemical.com
- Country:China
- ProdList:23541
- Advantage:58
-
Supplier:
Shenzhen Excellent Biomedical Technology Co.,Ltd.
- Tel: +undefined18318671572
- Email:sale@ex-biotech.com
- Country:China
- ProdList:1092
- Advantage:58
-
Supplier:
China National Standard Pharmaceutical Corporation Limited
- Tel: +8615391658522
- Email:overseasales@yongstandards.com
- Country:China
- ProdList:11922
- Advantage:58
-
Supplier:
Guangzhou CATO Research Chemicals Inc.
- Tel:+86-020-81960175-617<br/>+8615602392859
- Email:Intermediate@cato-chem.com
- Country:China
- ProdList:6335
- Advantage:58
-
Supplier:
Moxin Chemicals
- Tel: +8617320513646
- Email:Anna@molcoo.com
- Country:China
- ProdList:9684
- Advantage:58
-
Supplier:
Amadis Chemical Company Limited
- Tel:571-89925085
- Email:sales@amadischem.com
- Country:China
- ProdList:131957
- Advantage:58
-
Supplier:
QUALITY CONTROL SOLUTIONS LTD.
- Tel:0755-66853366;<br/>13670046396
- Email:ORDERS@QCSRM.COM
- Country:China
- ProdList:24342
- Advantage:58
- Supplier: S.Z. PhyStandard Bio-Tech. Co., Ltd.
- Tel:0755-4000505016<br/>13380397412
- Email:3001272453@qq.com
- Country:China
- ProdList:4999
- Advantage:50
97232-98-9, Cefuroxime Sodium EP Impurity GRelated Search:
- CefuroxiMe Axetil iMpurity C Cefuroxime Sodium EP Impurity F Δ2-Cefuroxime Axetil 7-Aminocephalosporanic acid (αZ)-α-(MethoxyiMino)-N-[(5aR,6R)-1,4,5a,6-tetrahydro-1,7-dioxo-3H,7H-azeto[2,1-b]furo[3,4-d][1,3]thiazin-6-yl]-2-furanacetaMide Cefuroxime 1-acetoxyethyl ester [6R-[6alpha,7beta(Z)]]-7-[2-furyl(methoxyimino)acetamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid Desethyl Acetate (E)-CefuroxiMe Axetil (Z)-alpha-(methoxyimino)furan-2-acetic acid Chlorosulfonyl isocyanate alpha-(methoxyimino)furan-2-acetic acid cefuracetime Cefuroxime sodium Triethyl phosphate Cefuroxime Sodium EP Impurity C
- 杂质对照品
- 化学试剂
- 头孢呋辛钠杂质
- 化药杂质-头孢呋辛钠
- 对照品-杂质对照品
- C17H17N3O8S
- 头孢呋辛杂质7(头孢呋辛SODIUMEP杂质G)
- (6R,7R)-3-(乙酰氧基甲基)-7-((E)-2-(呋喃-2-基)-2-(甲氧基亚氨基)乙酰氨基)-8-氧代-5-硫杂-1-氮杂双环[4.2 .0]辛-2-烯-2-羧酸 (头孢呋辛杂质)
- 头孢呋辛钠有关杂质 G
- 头孢呋辛钠EP杂质G
- 乙酰反式头孢呋辛钠
- 头孢呋辛杂质G(EP)
- 头孢呋辛EP杂质G
- 97232-98-9
- Cefuroxim Sodium EP Impurity G
- (6R,7R)-3-(Acetoxymethyl)-7-((E)-2-(furan-2-yl)-2-(methoxyimino)acetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid (Cefuroxime Impurity)
- 5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid, 3-[(acetyloxy)methyl]-7-[[2-furanyl(methoxyimino)acetyl]amino]-8-oxo-, [6R-[6α,7β(E)]]- (9CI)
- Cefuroxime Impurity 7/Cefuroxime EP Impurity G/(6R,7R)-3-(acetoxymethyl)-7-((E)-2-(furan-2-yl)-2-(methoxyimino)acetamido)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
- (6R,7R)-3-(acetyloxymethyl)-7-[[(2E)-2-(furan-2-yl)-2-methoxyiminoacetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid
- Cefuroxime Impurity 7(Cefuroxime Sodium EP Impurity G)
- Cefuroxime EP Impurity G
- Cefuroxime Sodium EP Impurity G