ChemicalBook > CAS DataBase List > Bromoacetyl chloride
Bromoacetyl chloride
Bromoacetyl chloride
- CAS No.22118-09-8
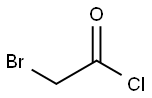
- Chemical Name:Bromoacetyl chloride
- CBNumber:CB2431011
- Molecular Formula:C2H2BrClO
- Formula Weight:157.39
- MOL File:22118-09-8.mol
Bromoacetyl chloride Property
- Boiling point 127-128 °C (lit.)
- Density 1.89 g/mL at 20 °C
- refractive index n
20/D 1.495 - storage temp. 2-8°C
- form Liquid
- color Clear yellow to brown
- BRN 1209323
- CAS DataBase Reference 22118-09-8(CAS DataBase Reference)
- NIST Chemistry Reference Bromoacetyl chloride(22118-09-8)
- UNSPSC Code 12352100
- NACRES NA.22
Safety
- Hazard Codes :C
- Risk Statements :14-34-37
- Safety Statements :26-36/37/39-45
- RIDADR :UN 3265 8/PG 2
- WGK Germany :3
- F :10-21
- HazardClass :8
- PackingGroup :II
- HS Code :29159000
-
NFPA 704:
0 3 0
-
Symbol(GHS)


- Signal wordDanger
- Hazard statements H314-H335
- Precautionary statements P261-P271-P280-P303+P361+P353-P304+P340+P310-P305+P351+P338
Bromoacetyl chloride Price
More Price(5)
- Brand: Sigma-Aldrich(India)
- Product number: 16120
- Product name : Bromoacetyl chloride
- Purity: ≥95% (GC)
- Packaging: 25ML
- Price: ₹7361
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 16120
- Product name : Bromoacetyl chloride
- Purity: ≥95% (GC)
- Packaging: 100ML
- Price: ₹25027.4
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 16120
- Product name : Bromoacetyl chloride
- Purity: ≥95% (GC)
- Packaging: 500ML
- Price: ₹107264.93
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: B0900
- Product name : Bromoacetyl Chloride
- Purity: min. 85.0 %
- Packaging: 25G
- Price: ₹4300
- Updated: 2022/05/26
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: B0900
- Product name : Bromoacetyl Chloride
- Purity: min. 85.0 %
- Packaging: 500G
- Price: ₹30200
- Updated: 2022/05/26
- Buy: Buy
Bromoacetyl chloride Chemical Properties,Usage,Production
- Chemical Properties Clear yellow liquid
-
Mechanism of action
Bromoacetyl chloride photodissociation has been interpreted as a typical process in which nonadiabatic effects play a dominant role. Stationary points (minima and saddle points) and minimum energy paths are characterized on the S0 and S1 potential energy surfaces. The five adiabatic excited electronic states are converted to a nonadiabatic representation using a quadruple-path nonadiabatic approach. The nonadiabatic potential energy matrix of the first five excited singlet states is constructed along several cuts of the potential energy hypersurface. The thermochemical properties of the photodissociation reaction and the comparison with experimental translational energy profiles strongly suggest that nonadiabatic effects dominate the C-Br cleavage, but the reaction proceeds along an energetically allowed nonadiabatic path to excited state products rather than being nonadiabatically inhibited. This conclusion is also supported by the low values of nonadiabatic coupling along the C-Br cleavage reaction path[1].
- Uses Bromoacetyl chloride was used in the synthesis of α,α-disubstituted thioisomünchnones. It was also used in the preparation of 1,3-dibromoacetone.
- General Description The competitive photodissociation of bromoacetyl chloride has been studied by ab initio methods.
- Precautions readily hydrolyzed with formation of HCl; corrosive; lachrymator. This reagent should only be handled in a fume hood.
- References [1] ROSENDO VALERO; Donald G T. Nonadiabatic effects in C-Br bond scission in the photodissociation of bromoacetyl chloride.[J]. Journal of Chemical Physics, 2006, 125 19: 194305. DOI:10.1063/1.2363991.
Bromoacetyl chloride Preparation Products And Raw materials
Raw materials
Preparation Products
Global(203)Suppliers
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5854
- Advantage:58
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Shanghai Time Chemicals CO., Ltd.
- Tel:+86-021-57951555<br/>+8617317452075
- Email:jack.li@time-chemicals.com
- Country:China
- ProdList:1803
- Advantage:55
-
Supplier:
Nanjing ChemLin Chemical Industry Co., Ltd.
- Tel:025-83697070
- Email:product@chemlin.com.cn
- Country:CHINA
- ProdList:3009
- Advantage:60
-
Supplier:
Speranza Chemical Co., Ltd.
- Tel:+86-75521030354<br/>+86-18688942810
- Email:sales@speranzachem.com
- Country:China
- ProdList:927
- Advantage:55
-
Supplier:
Win-Win Chemical CO., Limited
- Tel:0086-577-64498589
- Email:sales@win-winchemical.com
- Country:CHINA
- ProdList:998
- Advantage:58
-
Supplier:
Changzhou Ansciep Chemical Co., Ltd.
- Tel:+86 519 86305871
- Email:sales@ansciepchem.com
- Country:CHINA
- ProdList:4241
- Advantage:58
-
Supplier:
Jinan Carbotang Biotech Co.,Ltd.
- Tel: +8615866703830
- Email:figo.gao@foxmail.com
- Country:China
- ProdList:8497
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
22118-09-8, Bromoacetyl chlorideRelated Search:
- Ammonium chloride Sodium chloride Chloroacetyl chloride Acetyl chloride Calcium chloride Oxalyl chloride Choline chloride Phenoxyacetyl chloride Zinc chloride Thionyl chloride Acetoxyacetyl chloride Bromoacetyl bromide Trichloroacetyl chloride Potassium chloride Phenylacetyl chloride Bromoethane Bromoethane triphenylphosphine salt 2-Bromopropionyl chloride
- Organic Building Blocks
- Carbonyl Compounds
- Acid Halides
- 高端化学
- 原料药
- 杀菌剂中间体
- 三唑类杀菌剂
- 农药中间体
- 合成
- Building Blocks
- Carbonyl Compounds
- Organic Building Blocks
- Acid Halides
- BrCH2COCl
- C2H2BrClO
- PERFEMIKER]溴乙酰氯,98%
- 溴乙酰氯 500GR
- 溴乙酰氯 5G
- 溴乙酰氯
- 22118-09-8
- monobromoacetic acid chloride
- TIANFU CHEM 22118-09-8 Bromoacetyl chloride
- Acetyl chloride, 2-bromo-
- Bromoacetyl Chloride >
- Acetyl chloride, bromo-(6CI,7CI,8CI,9CI)
- JACS-22118-09-8
- BroMoacetyl chloride >=95% (GC)
- 2-bromoethanoyl chloride
- Bromoacetic acid chloride
- monobromo acetyl chloride
- Acetyl chloride, bromo-
- BROMOACETYL CHLORIDE, (CONTAINS VARYING AMOUNTS OF CHLOROACETYL CHLORIDE): 65%
- BROMOACETYL CHLORIDE