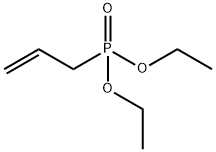
ChemicalBook > CAS DataBase List > Diethyl allylphosphonate
Diethyl allylphosphonate
Diethyl allylphosphonate
- CAS No.1067-87-4
- Chemical Name:Diethyl allylphosphonate
- CBNumber:CB2385035
- Molecular Formula:C7H15O3P
- Formula Weight:178.17
- MOL File:1067-87-4.mol
Diethyl allylphosphonate Property
- Boiling point 46 °C/0.35 mmHg (lit.)
- Density 1,035 g/cm3
- bulk density 1.022g/mL
- vapor pressure 55.2Pa at 25℃
- refractive index n
20/D 1.4340(lit.) - Flash point 224 °F
- form Liquid
- color Clear colorless
- InChI InChI=1S/C7H15O3P/c1-4-7-11(8,9-5-2)10-6-3/h4H,1,5-7H2,2-3H3
- InChIKey YPJHXRAHMUKXAE-UHFFFAOYSA-N
- SMILES P(CC=C)(=O)(OCC)OCC
- LogP 1.33 at pH7
- Surface tension 56.4-60.9mN/m at 1g/L and 19℃
- CAS DataBase Reference 1067-87-4(CAS DataBase Reference)
- UNSPSC Code 12352108
- NACRES NA.22
Safety
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H315-H319-H335
- Precautionary statements P261-P264-P271-P280-P302+P352-P305+P351+P338
Diethyl allylphosphonate Price
More Price(3)
- Brand: Sigma-Aldrich(India)
- Product number: 565415
- Product name : Diethyl allylphosphonate
- Purity: 98%
- Packaging: 5G
- Price: ₹7978.03
- Updated: 2022/06/14
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: D3069
- Product name : Diethyl Allylphosphonate
- Purity: min. 95.0 %
- Packaging: 1G
- Price: ₹3000
- Updated: 2022/05/26
- Buy: Buy
- Brand: TCI Chemicals (India)
- Product number: D3069
- Product name : Diethyl Allylphosphonate
- Purity: min. 95.0 %
- Packaging: 5G
- Price: ₹7400
- Updated: 2022/05/26
- Buy: Buy
Diethyl allylphosphonate Chemical Properties,Usage,Production
- Chemical Properties clear colorless liquid
-
Uses
Reactant for:
- Copolymerization of phosphonated allyl monomers and maleic anhydride
- Enantioselective synthesis of solamin type mono-THF acetogenins
- RCM reaction yielding oxaphospholene and oxaphosphinene heterocycles
- Synthesis of spongistatin 2 using Wittig coupling
- Stereoselective synthesis of pentacyclic furanosteroids
- Preparation of protected polyhydroxylated β -amino acid constitutents of microsclerodermins
-
Reactions
To a stirred solution of diethyl allylphosphonate (582.5 mg, 3.0 mmol, 1.5 equiv) in dry THF (25 mL) was added NaH (60% dispersion in mineral oil, 120 mg, 3.0 mmol, 1.5 equiv) portion wise under nitrogen atmosphere at 0°C. After 15 min, aryl/alkyl aldehyde 14a–x, 14z (2.0 mmol) in dry THF was added to the reaction mixture and stirred at 0°C until the completion (monitored by TLC) of reaction. It was then quenched with saturated aq. NH4Cl and the solution was extracted with EtOAc (2×20 mL). The combined organic layers were dried (Na2SO4) and concentrated. The residue was purified by silica gel column chromatography using petroleum ether/EtOAc (9:1, common for all compounds unless specified) as eluent to afford (E)-1-aryl/alkylbutadienes 7a–x, 7z.
The CM reaction of 2 with dimethyl vinylphosphonate (3a) and diethyl allylphosphonate (3b) under a classic thermal condition with 3 mol % of H-G II at 40 °C.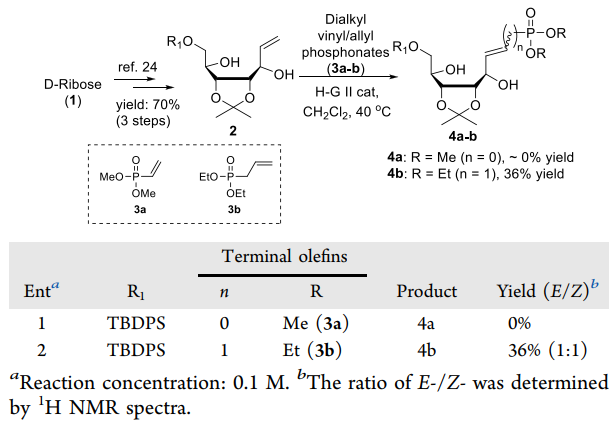
-
Synthesis Reference(s)
Synthetic Communications, 22, p. 2219, 1992 DOI: 10.1080/00397919208019075
Synthesis, p. 563, 1986 DOI: 10.1055/s-1986-31704 - reaction suitability reaction type: C-C Bond Formation
-
References
[1] CHOUDHARY P, RANJAN R S, FERNANDES R. Cu-Catalyzed Coupling of Sulfonyl Chlorides with Alkenes: Synthesis of Dienyl Sulfones and β-Chlorosulfones[J]. European Journal of Organic Chemistry, 2024, 28 1. DOI:10.1002/ejoc.202401077.
[2] SE MYEONG CHOI, JONG HYUN CHO* Direct Synthesis of Aryloxy Phosphonamidate Nucleotide Prodrugs Using the Cross Metathesis Assisted by Ultrasonic Irradiation[J]. Organic Letters, 2024, 26 23: 4841-4846. DOI:10.1021/acs.orglett.4c00094.
[3] ELIZABETH I. PARKINSON. Fosmidomycin biosynthesis diverges from related phosphonate natural products[J]. Nature chemical biology, 2019, 15 11: 1049-1056. DOI:10.1038/s41589-019-0343-1.
[4] PAWE? MITU?A C. W. Synthesis of a series of new racemic [2,3-bis(acyloxy)propyl]phosphonocholines[J]. Arkivoc, 2012, 2012 1. DOI:10.3998/ARK.5550190.0013.416.
Diethyl allylphosphonate Preparation Products And Raw materials
Raw materials
Preparation Products
Global(136)Suppliers
-
Supplier:
XINXIANG RUNYU MATERIAL CO., LTD.
- Tel:+86-13592593621;<br/>+8613592593621
- Email:sales@runvmat.com
- Country:China
- ProdList:408
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-29-81148696<br/>+86-15536356810
- Email:1022@dideu.com
- Country:China
- ProdList:3882
- Advantage:58
-
Supplier:
ATK CHEMICAL COMPANY LIMITED
- Tel:+undefined-21-51877795
- Email:ivan@atkchemical.com
- Country:China
- ProdList:33024
- Advantage:60
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:factory@coreychem.com
- Country:China
- ProdList:29808
- Advantage:58
-
Supplier:
Honest Joy Holdings Limited
- Tel:0755-36694831<br/>+8613717124449
- Email:sale@feiyang.com.cn
- Country:China
- ProdList:300
- Advantage:58
-
Supplier:
Dayang Chem (Hangzhou) Co.,Ltd.
- Tel:+86-0571-88938639<br/>+8617705817739
- Email:info@dycnchem.com
- Country:China
- ProdList:52849
- Advantage:58
-
Supplier:
CD Chemical Group Limited
- Tel: +8615986615575
- Email:info@codchem.com
- Country:China
- ProdList:20342
- Advantage:58
-
Supplier:
Henan Alfa Chemical Co., Ltd
- Tel: +8615838112936
- Email:alfa10@alfachem.cn
- Country:China
- ProdList:13194
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531151365
- Email:mina@chuanghaibio.com
- Country:China
- ProdList:18137
- Advantage:58
-
Supplier:
PT CHEM GROUP LIMITED
- Tel:+86-85511178;<br/>+86-85511178;
- Email:peter68@ptchemgroup.com
- Country:China
- ProdList:35425
- Advantage:58
1067-87-4, Diethyl allylphosphonateRelated Search:
- Diethyl allylmalonate 1-allylhydrazine hydrochloride ALLYLDIPHENYLPHOSPHINE Allyl methyl carbonate ALLYLDIPHENYLPHOSPHINE OXIDE Dimethyl allylmalonate Allyl Benzyl Ether Allylbenzene ALLYLUREA ALLYL PHENYL SULFIDE Allylthiourea ALLYLCHLORO[1,3-BIS(2,6-DI-I-PROPYLPHENYL)-4,5-DIHYDROIMIDAZOL-2-YLIDENE]PALLADIUM (II) ALLYL METHYL ETHER 2-Allyloxyethanol ALLYL IODIDE Allylboronic acid pinacol ester Allylamine hydrochloride TRIETHYL 4-PHOSPHONOCROTONATE
- Olefination
- Horner-Wadsworth-Emmons Reagents
- C-C Bond Formation
- 高端化学
- 其他生化试剂
- 单体
- Olefination
- Horner-Wadsworth-Emmons Reagents
- Synthetic Reagents
- C-C Bond Formation
- C7H15O3P
- CH2CHCH2POOC2H52
- C2H5O2POCH2CHCH2
- 二乙基 烯丙基磷酸酯
- 烯丙基膦酸二乙基酯
- 烯丙基膦酸二乙酯, 95+%
- 烯丙基膦酸二乙酯
- 烯丙基磷酸二乙酯
- 1067-87-4
- Diethyl?P-2-propen-1-ylphosphonate
- DiethylAllylphosphonate>
- Phosphonic acid, P-2-propen-1-yl-, diethyl ester
- 3-diethoxyphosphorylprop-1-ene
- Allylphosphonic acid diethyl
- 2-Propenylphosphonic acid diethyl
- (2-Propenyl)phosphonic acid diethyl ester
- Diethyl allylphosphonate, 95+%
- Diethylallylphosphonate, 97 %
- ALLYLPHOSPHONIC ACID DIETHYL ESTER
- AURORA KA-1466
- DIETHYL (1-PROPENYL)PHOSPHONATE
- DIETHYL(2-PROPENYL)PHOSPHONATE
- DIETHYL-(2-PROPENYL)-PHOSPHONAT
- DIETHYL ALLYLPHOSPHONATE
- DIETHYL-(ALLYLPHOSPHONAT)
- DIETHYL(PROP-2-(E)-ENYL)PHOSPHONATE
- DIETHYL(PROP-1(E)-ENYL)PHOSPHONATE