ChemicalBook > CAS DataBase List > Naloxone hydrochloride
Naloxone hydrochloride
Naloxone hydrochloride
- CAS No.357-08-4
- Chemical Name:Naloxone hydrochloride
- CBNumber:CB1409273
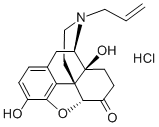
- Molecular Formula:C19H22ClNO4
- Formula Weight:363.84
- MOL File:357-08-4.mol
Naloxone hydrochloride Property
- Melting point 200-2050C
- RTECS QD2275000
- storage temp. 2-8°C
- solubility ethanol: 3.3 mg/mL stable for several months refrigerated if protected from light.
- form powder
- color white to off-white
- Water Solubility Soluble in water (73 mg/ml), ethanol (3.3 mg/ml), methanol (50 mg/ml) and dimethyl sulfoxide (73 mg/ml). Insoluble in ether
- Stability Stable for 1 year from date of purchase as supplied. Solutions in DMSO or distilled water may be stored at -20°C for up to 2 months.
- InChI InChI=1/C19H21NO4.ClH/c1-2-8-20-9-7-18-15-11-3-4-12(21)16(15)24-17(18)13(22)5-6-19(18,23)14(20)10-11;/h2-4,14,17,21,23H,1,5-10H2;1H/t14-,17+,18+,19-;/s3
- InChIKey RGPDIGOSVORSAK-ASLVLMPBNA-N
- SMILES [C@@]123CCN(CC=C)[C@@H]4CC5=CC=C(O)C(O[C@H]1C(CC[C@]24O)=O)=C35.Cl |&1:0,7,16,20,r|
- CAS DataBase Reference 357-08-4(CAS DataBase Reference)
- FDA UNII F850569PQR
- NCI Drug Dictionary naloxone hydrochloride
Safety
- Hazard Codes :Xi
- Risk Statements :36/37/38
- Safety Statements :22-24/25-26-36/37/39-36
- WGK Germany :3
-
NFPA 704:
0 3 0
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H315-H319-H335
- Precautionary statements P261-P280a-P304+P340-P305+P351+P338-P405-P501a
Naloxone hydrochloride Chemical Properties,Usage,Production
- Description Naloxone (hydrochloride) (CRM) (Item No. ISO60191) is a certified reference material categorized as an opioid antagonist. Formulations containing naloxone have been used as antidotes for opioid overdose and the prevention of overdose. They have also been used in combination with buprenorphine (Item Nos. ISO60178 | 14025) in the treatment of opiate addiction and in pain management. This product is intended for research and forensic applications.
- Chemical Properties Crystalline Solid
- Originator Narcan,Du Pont,US,1971
- Uses Naloxone Hydrochloride is a specific opioid antagonist. Narcotic antagonist.
- Uses A nonspecific opiate receptor antagonist that displays anitnociceptive effects
- Uses Opioate antidote;
- Definition ChEBI: A hydrochloride resulting from the formal reaction of equimolar amounts of naloxone and hydrogen chloride. A specific opioid antagonist, it is used to reverse the effects of opioids, both following their use of opioids during surgery and in cases of known r suspected opioid overdose.
-
Manufacturing Process
10 grams of 14-hydroxydihydromorphinone (oxymorphone) was converted
into its diacetate by warming it on the steam bath with 80 cc of acetic
anhydride for about 2 hours. The acetic anhydride was removed on the water
bath under a vacuum of about 30 mm absolute pressure. The melting point of
the residue was 220°C. The residue was taken up in 100 cc of chloroform. An
equal amount by weight of cyanogen bromide was added and the mixture was
refluxed at about 60°C for about 5 hours. After refluxing, the mixture was
washed with 100 cc of a 5% aqueous hydrochloric acid solution, dried over
sodium sulfate and the chloroform removed by evaporation under a vacuum of
about 30 mm. The residue had a melting point of 240°C.
The residue was then heated at about 90°C for 16 hours on a steam bath with 300 cc of 20% aqueous hydrochloric acid solution, and treated with a small amount, e.g., 1 gram of charcoal. The hydrochloric acid was then removed under a vacuum of 15 mm, the residue dissolved in 30 cc of water and precipitated by the addition of 2.4 cc of concentrated aqueous ammonia. The precipitate was filtered off and dried. It consists of 14- hydroxydihydronormorphinone. It is soluble in ethanol.
The 14-hydroxydihydronormorphinone was suspended in 200 cc of pure ethyl alcohol, half its weight of sodium bicarbonate and half its weight of allyl bromide added and the resulting mixture was refluxed at about 75°C for 48 hours. The solution was cooled, e.g., to 10°C and filtered and the alcohol removed under a vacuum of 30 mm. The residue was dissolved in chloroform and filtered. The chloroform was removed under a vacuum of 30 mm and the residue was crystallized from ethylacetate. The crystallized product, N-allyl- 1,4-hydroxydihydronormorphinone, has a melting point of 184°C, is soluble in chloroform and insoluble in petroleum ether. The yield amounts to 20% based on the weight of the reacted 14-hydroxydihydromorphinone. - brand name Narcan (Bristol-Myers Squibb); Narcan (Endo).
- Therapeutic Function Narcotic antagonist
- Biological Activity Opioid antagonist.
- Veterinary Drugs and Treatments Naloxone is used in veterinary medicine almost exclusively for its opiate reversal effects, but the drug is being investigated for treating other conditions (e.g., septic, hypovolemic or cardiogenic shock). Naloxone may also be employed as a test drug to see if endogenous opiate blockade will result in diminished tail chasing or other self-mutilating behaviors. It, potentially, could be useful for treating overdoses of clonidine or the CNS effects of benzodiazepines (ivermectin?), but more research is necessary before recommending its use.
- Metabolism Naloxone hydrochloride is rapidly metabolised in the liver, mainly by conjugation with glucuronic acid to naloxone-3-glucuronide, which is excreted in the urine
- storage Room temperature
- References 1) Le Bourdonnec?et al., (2008),?Novel trans-3,4-dimethyl-4-(3-hydroxyphenyl)piperidines as mu opioid receptor antagonists with improved opioid receptor selectivity profiles: Bioorg. Med. Chem. Lett..?18?2006 2) Boyer?et al.?(2012),?Management of opioid analgesic overdose; New Engl. J. Med.?367?146 3) Wang?et al.?(2017),?Opioids and opioid receptors orchestrate wound repair;?Transl. Res.?185?13 4) Wright and Rodgers (2013),?Low dose naloxone attenuates the pruritic but not anorectic response to rimonabant in male rats; Psychopharmacology (Berl.)?226?415
Naloxone hydrochloride Preparation Products And Raw materials
Raw materials
Preparation Products
Global(268)Suppliers
-
Supplier:
Guangzhou Tengyue Chemical Co., Ltd.
- Tel:+86-86-18148706580<br/>+8618826483838
- Email:evan@tyvovo.com
- Country:China
- ProdList:146
- Advantage:58
-
Supplier:
Nantong Guangyuan Chemicl Co,Ltd
- Tel: +undefined17712220823
- Email:admin@guyunchem.com
- Country:China
- ProdList:615
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
Shanxi Naipu Import and Export Co.,Ltd
- Tel:+86-13734021967<br/>+8613734021967
- Email:kaia@neputrading.com
- Country:China
- ProdList:1001
- Advantage:58
-
Supplier:
Shanghai Zheyan Biotech Co., Ltd.
- Tel:18017610038
- Email:zheyansh@163.com
- Country:CHINA
- ProdList:3619
- Advantage:58
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Standardpharm Co. Ltd.
- Tel:86-714-3992388
- Email:overseasales1@yongstandards.com
- Country:United States
- ProdList:14332
- Advantage:58
-
Supplier:
Chongqing Chemdad Co., Ltd
- Tel:+86-023-6139-8061<br/>+86-86-13650506873
- Email:sales@chemdad.com
- Country:China
- ProdList:39894
- Advantage:58
-
Supplier:
CONIER CHEM AND PHARMA LIMITED
- Tel: +8618523575427
- Email:sales@conier.com
- Country:China
- ProdList:49732
- Advantage:58
-
Supplier:
Antai Fine Chemical Technology Co.,Limited
- Tel: 18503026267
- Email:info@antaichem.com
- Country:CHINA
- ProdList:9636
- Advantage:58
357-08-4, Naloxone hydrochloride Related Search:
- Topotecan hydrochloride Pyridine hydrochloride 4-Hydroxyaniline hydrochloride D-Glucosamine hydrochloride Methoxyammonium chloride Triethylamine hydrochloride Resin epoxy Naloxone Impurity 3(Naloxone EP Impurity C) IDDUCSVQXOGMNE-GVVUEQADSA-N 2,2-Bisnaloxone Naloxone 5,6,7,8-TETRAHYDRO-1-NAPHTHOL 1-PHENYL-3-METHYL-3-PENTANOL 4-N-PROPYLPIPERIDINE 4-BUTYLPIPERIDINE NALOXONE HYDROCHLORIDE, DIHYDRATE RADICININ FROM ALTERNARIA CHRYSANTHEMI 4-(phenoxymethyl)piperidinium chloride
- Opioids
- Opioid receptor and opioid-like receptor
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Drug Analogues
- Chiral Reagents
- API
- 化工中间体
- 抑制剂
- 药靶配体
- 白色粉末
- 科研精品试剂
- 化学试剂
- 化工原料
- 原料
- 标准品 -中药标准品
- 其他原料药
- 原料药
- 医药原料药-科研原料
- 神经信号
- 医药原料
- 小分子抑制剂
- C19H21NO4HClC19H22ClNO4
- C19H22ClNO4
- C19H21NO4HCl
- C19H21NO4HClH2O
- NALOXONE HYDROCHLORIDE,OPIOID拮抗剂
- ZELAVESPIB
- 盐酸纳洛酮/NALOXONE HYDROCHLORIDE
- 盐酸纳呋拉啡杂质IMP28
- 盐酸纳洛酮(二水物)
- 纳洛酮杂质9
- 盐酸纳洛酮对照品
- 纳洛酮盐酸盐
- 盐酸纳络酮
- 盐酸纳诺酮
- 盐酸纳洛酮/17-烯丙基-4,5A-环氧基-3,14-二羟基吗啡喃-6-酮盐酸盐
- (-)-纳洛酮 盐酸盐
- 17-烯丙基-4,5α-环氧-3,14-二羟基吗啡烷-6-酮
- 17-烯丙基-4,5a-环氧基-3,14-二羟基吗啡喃-6-酮盐酸盐
- 盐酸纳洛酮
- 357-08-4
- Zelavespib
- Naloxone hydrochloride in methanol
- Naloxone Hydrochloride hydrate
- narcan
- NALOXONE HCL
- Naloxone hydrochlori
- Naloxone hydrochloride USP/EP/BP
- Naloxone (hydrochloride) (CRM)
- Naloxone hydrochloride (controlled)
- (5α)-4,5-Epoxy-3,14-dihydro-17-(2-propenyl)morphinan-6-onehydrochloride
- (5alpha)-4,5-Epoxy-3,14-dihydroxy-17-(2-propenyl)morphinan-6-one hydrochloride
- Normorphinone, N-allyl-7,8-dihydro-14-hydroxy-, hydrochloride (7CI)
- N-Allyldihydro-14-hydroxynormorphinone hydrochloride
- Morphinan-6-one, 4,5-epoxy-3,14-dihydroxy-17-(2-propenyl)-, hydrochloride, (5a)- (9CI)
- Morphinan-6-one, 17-allyl-4,5a-epoxy-3,14-dihydroxy-, hydrochloride (8CI)
- l-Naloxone hydrochloride