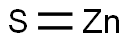ChemicalBook > CAS DataBase List > Zinc sulfide
Zinc sulfide
Zinc sulfide
- CAS No.1314-98-3
- Chemical Name:Zinc sulfide
- CBNumber:CB0733609
- Molecular Formula:SZn
- Formula Weight:97.46
- MOL File:1314-98-3.mol
Zinc sulfide Property
- Melting point 1700°C
- Boiling point 1185°C (estimate)
- Density 4.1 g/mL at 25 °C (lit.)
- solubility insoluble in H2O, ethanol; soluble in dilute acid
- form powder
- color white to faint yellow
- Specific Gravity 4.1
- Water Solubility Soluble in acids. insoluble in water.
- Crystal Structure CuCl type
- crystal system Cube
- Merck 14,10160
- Solubility Product Constant (Ksp) pKsp: α-ZnS 23.80;β-ZnS 21.60
- Space group F43m
- Lattice constant
a/nm b/nm c/nm α/o β/o γ/o V/nm3 0.53829 0.53829 0.53829 90 90 90 0.156 - Dielectric constant 1.7(Ambient)
- Stability Stable. May react with water to give toxic hydrogen sulfide. Incompatible with acids, strong oxidizing agents. Air and moisture sensitive.
- InChI InChI=1S/S.Zn
- InChIKey WGPCGCOKHWGKJJ-UHFFFAOYSA-N
- SMILES S=[Zn]
- CAS DataBase Reference 1314-98-3(CAS DataBase Reference)
- Indirect Additives used in Food Contact Substances ZINC SULFIDE
- FDA 21 CFR 175.105; 177.2600; 178.3297; 178.3570; 310.545
- EWG's Food Scores 2
- FDA UNII KPS085631O
- EPA Substance Registry System Zinc sulfide (1314-98-3)
- UNSPSC Code 12352200
- NACRES NA.23
Safety
- Risk Statements :31
- Safety Statements :50A
- WGK Germany :3
- RTECS :ZH5400000
- TSCA :Yes
- HS Code :28309085
- Hazardous Substances Data :1314-98-3(Hazardous Substances Data)
-
NFPA 704:
0 1 0
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
Zinc sulfide Price
More Price(23)
- Brand: Sigma-Aldrich(India)
- Product number: 769355
- Product name : Zinc sulfide
- Purity: sublimed, granular, 1-4?mm
- Packaging: 50G
- Price: ₹13953.43
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 769347
- Product name : Zinc sulfide
- Purity: pellets, diam. × thickness 10?mm × 6.2?mm
- Packaging: 50G
- Price: ₹9677.55
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 333271
- Product name : Zinc sulfide
- Purity: pieces, 3-12?mm, 99.9% trace metals basis
- Packaging: 50G
- Price: ₹7220.28
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 244627
- Product name : Zinc sulfide
- Purity: powder, 10?μm, 99.99% trace metals basis
- Packaging: 25G
- Price: ₹3344.93
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 244627
- Product name : Zinc sulfide
- Purity: powder, 10?μm, 99.99% trace metals basis
- Packaging: 100G
- Price: ₹9038.88
- Updated: 2022/06/14
- Buy: Buy
Zinc sulfide Chemical Properties,Usage,Production
-
Occurrence and Uses
Zinc sulfide occurs in nature in two crystalline forms, the minerals, wurtzite, and sphalerite. Sulfide ore is the principal zinc mineral.

The most important use of Zinc sulfide is as a pigment. As lithopone, a mixture with barium sulfate, it forms a low gloss interior house paint. The pigment, “mineral white” is made by combining zinc sulfide with zinc oxide. Zinc sulfide is incorporated into phosphors to produce luminescence when irradiated with light. It is used in making luminous dials, x-ray and television screens, and fluorescent lights. Also, it is used in making white and opaque glass and as a base for color lakes (which consist of an organic pigment with an inorganic carrier). - Physical Properties Zinc sulfide is white to gray-white or pale yellow powder. It exists in two crystalline forms, an alpha (wurtzite) and a beta (sphalerite). The wurtzite form has hexagonal crystal structure; refractive index 2.356; density 3.98 g/cm3; melts at 1,700°C; practically insoluble in water, about 6.9 mg/L; insoluble in alkalis; soluble in mineral acids. The sphalerite form arranges in cubic crystalline state; refractive index 2.368; density 4.102 g/cm3; changes to alpha form at 1,020°C; practically insoluble in water, 6.5 mg/L; soluble in mineral acids, insoluble in alkalis. When zinc sulfide contains water, it slowly oxidizes to sulfate on exposure to air.
-
Uses
Zinc sulfide is well known as an optical coating material for its high refractive index (~2.35 at 500 nm) and very broad transmittance range from 400 nm up to 14 µm. It allows for coatings in the IR and VIS range with good environmental durability, and can be evaporated rapidly from e-beam and resistance heated sources. It is used as a semiconductor and in photo optic applications.
Copperactivated zinc sulfides are the most widely used phosphors for safety purposes.
Zinc sulfide and white lead are sometimes used in coating materials because of their fungicidal properties and to neutralize acid to protect against corrosion. -
Production
Zinc sulfide is mined from natural deposits and concentrated by various processes.
Also, zinc sulfide may be prepared in the laboratory by passing hydrogen sulfide through an aqueous solution of a soluble zinc salt, such as zinc chloride or zinc nitrate. The precipitate is filtered, washed, and dried. - Chemical Properties Yellowish-white powder. ZnS exists in two crystalline forms, α (wurtzite) and β (sphalerite). Stable if kept dry. α: d 3.98. β: d 4.102, changes to α form at 1020C. Sublimes at 1180C. Soluble in acids; insoluble in water.
- Uses Zinc sulfide (ZnS) is used as a pigment and to make white glass, rubber, and plastics. It is an ingredient in pesticides, luminous paints, and X-ray and television screens.
- Uses Pigment for paints, oilcloths, linoleum, leather, dental rubber, etc., especially in the form of lithopone; mixed with ZnO as "mineral white." Anhydrous zinc sulfide is used in x-ray screens and with a trace of a radium or mesothorium salt in luminous dials of watches; also television screens.
- Uses Zinc Sulfide is used as a friction material to carry sulfur, which hardens rotor surfaces and thereby reduces wear. Also in printing inks, UV-hardened systems, powder coatings, adhesives, insulating & sealing compounds, thermoplastic pigmentation, thermosets, flame resistant plastics, glass-fiber reinforced plastics, pigment concentrates, elastomers,. textile fibers, paper, mastics, lubricants, electroluminescent lamps, infrared windows, domes and optical elements.
- Production Methods Production is similar to that of lithopone. A Na2S solution is mixed with a zinc salt solution under precisely controlled conditions. The resulting zinc sulfide precipitate is calcined and processed to give the finished product. Na2S + ZnSO4 →ZnS + Na2SO4.
- Definition zinc sulphide: A yellow-whitewater-soluble solid, ZnS. It occursnaturally as sphalerite (see also zincblende) and wurtzite. The compoundsublimes at 1180°C. It is used as apigment and phosphor.
- General Description A yellowish-white powder in a liquid. Insoluble in water and denser than water. Primary hazard is to the environment. Immediate steps should be taken to limit spread to the environment. Easily penetrates the soil to contaminate groundwater and nearby waterways.
- Air & Water Reactions Insoluble in water.
- Reactivity Profile These sulfides are rather inert, dissolve into acid, insoluble in water and alkalis.
- Health Hazard Inhalation of material may be harmful. Contact may cause burns to skin and eyes. Inhalation of Asbestos dust may have a damaging effect on the lungs. Fire may produce irritating, corrosive and/or toxic gases. Some liquids produce vapors that may cause dizziness or suffocation. Runoff from fire control may cause pollution.
- Fire Hazard Some may burn but none ignite readily. Containers may explode when heated. Some may be transported hot.
-
reaction suitability
core: zinc
reagent type: catalyst -
Industrial uses
Zinc sulfide(Sachtolith) is mainly used in plastics. Functional properties such as lightening and hiding power are criteria for the use of Sachtolith. It has proved to be very useful for coloring many thermoplasts. During the dispersion process it does not cause abrasion of metallic production machinery or adversely effect the polymer, even at high operating temperatures or during multistage processing. Even ultrahigh molecular mass thermoplasts can be colored without problems. In glass-fiber-reinforced plastics, the soft texture of Sachtolith prevents mechanical fiber damage during extrusion. Sachtolith is also used as a dry lubricant during the fabrication of these materials.
The low abrasiveness of Sachtolith prolongs the operating life of stamping tools used in the manufacture of industrial rubber articles. The lightfastness and ageing resistance of many elastomers are improved by using Sachtolith. It is also used as a dry lubricant for roller and plain bearings, and as a white pigment for greases and oils. -
Toxicology
The use of zinc sulfide and barium sulfate in contact with foods is permitted by the FDA (United States) and in most European countries. Some restrictions apply in France, Italy, the United Kingdom, and Czechoslovakia.
Soluble zinc is toxic in large amounts, but the human body requires small quantities (10–15 mg d−1) for metabolism. Zinc sulfide is harmless in the human due to its low solubility. The acid concentration in the stomach and the rate of dissolution following ingestion are not sufficient to produce physiologically significant quantities of soluble zinc. LD50 values in the rat exceed 15 g kg−1. No cases of poisoning or chronic damage to health have been observed in the manufacture of zinc sulfide pigments.
Zinc sulfide Preparation Products And Raw materials
Raw materials
Preparation Products
Global(358)Suppliers
-
Supplier:
Suzhou Actchem Co., Ltd.
- Tel: +8618762124502
- Email:actchem@qq.com
- Country:China
- ProdList:27
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Shaanxi Dideu Medichem Co. Ltd
- Tel:+86-29-81148696<br/>+86-15536356810
- Email:1022@dideu.com
- Country:China
- ProdList:3882
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12811
- Advantage:58
-
Supplier:
Hebei Yanxi Chemical Co., Ltd.
- Tel: +8617531153977
- Email:allison@yan-xi.com
- Country:China
- ProdList:5854
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co,.LTD
- Tel: +86-13131129325
- Email:sales1@chuanghaibio.com
- Country:China
- ProdList:5869
- Advantage:58
-
Supplier:
Hebei Fengjia New Material Co., Ltd
- Tel:+86-0311-87836622<br/>+86-18712993135
- Email:sales01@tairunfaz.com
- Country:China
- ProdList:8051
- Advantage:58
-
Supplier:
Henan Fengda Chemical Co., Ltd
- Tel:+86-371-86557731<br/>+86-13613820652
- Email:info@fdachem.com
- Country:China
- ProdList:20235
- Advantage:58
-
Supplier:
Hebei Saisier Technology Co., LTD
- Tel:+86-18400010335<br/>+86-18034520335
- Email:admin@hbsaisier.cn
- Country:China
- ProdList:1015
- Advantage:58
-
Supplier:
Hebei Zhuanglai Chemical Trading Co.,Ltd
- Tel: +8613343047651
- Email:admin@zlchemi.com
- Country:China
- ProdList:3692
- Advantage:58
Related articles
1314-98-3, Zinc sulfideRelated Search:
- Zinc sulphate Zinc sulfate monohydrate Zinc carbonate hydroxide Zinc oxide Zinc fluoride Zinc dibenzyldithiocarbamate Zinc disodium EDTA ZINC OXALATE Zinc borate Zinc Phytate Zinc benzenesulfinate dihydrate Zinc chloride zinc bis[p-toluenesulphinate] ZINC CARBONATE BASIC Zinc hydroxide ZINC AMMONIUM CHLORIDE Zinc ethylphenyl dithiocarbamate Zinc acetate
- Inorganics
- Chemical Synthesis
- Micro/Nanoelectronics
- Electronic Chemicals
- Semiconductor Grade ChemicalsChemical Synthesis
- Zinc
- Metal and Ceramic Science
- Materials Science
- ChalcogenidesChemical Synthesis
- Catalysis and Inorganic Chemistry
- metal chalcogenides
- 日用化工
- 阻燃剂
- 新型无机功能材料
- 硫化物粉体
- 无机材料
- 其它原料及中间体
- 金属催化剂
- 无机化工原料-无机盐
- 其他化工产品
- 有机类
- 高端化学
- 大化工产品
- 化工
- 原料药
- 硫化物粉体-硫化锌(ZnS)
- 无机产品
- 精细化工品
- 电子材料
- 化工品
- 有机化工原料
- 无机物
- 光学
- 化工原料
- 无机化工原料
- 医药、农药及染料中间体
- 硫化物粉体-硫化锌
- 硫化物-硫化锌
- 其他原料
- 生化试剂
- 无机化工原料
- 生化试剂-其他化学试剂
- 硫化物
- 锌
- 通用试剂
- 重金属
- 催化和无机化学
- 无机盐
- 无机化工产品
- Co硫化物及硫酸盐
- 镀膜材料
- ZnSH2O
- ZnS
- 硫化锌 三氧化二锑的替代品 阻燃剂,对标德国撒哈利本
- 硫化锌HD-S
- 硫化锌粉99.999%
- 99.999%硫化锌
- 99.99%硫化锌