ChemicalBook > CAS DataBase List > Pramipexole
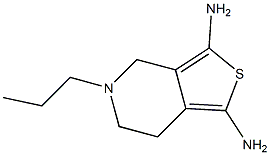
Pramipexole
Pramipexole
- CAS No.104632-26-0
- Chemical Name:Pramipexole
- CBNumber:CB0486178
- Molecular Formula:C10H17N3S
- Formula Weight:211.33
- MOL File:104632-26-0.mol
Pramipexole Property
- Melting point 288-290°C
- Boiling point 378.0±42.0 °C(Predicted)
- Density 1.17±0.1 g/cm3(Predicted)
- storage temp. Keep in dark place,Inert atmosphere,2-8°C
- solubility DMSO (Slightly), Methanol (Slightly)
- form Solid
- pka 9.47±0.20(Predicted)
- color White to Off-White
- InChI InChI=1S/C10H17N3S/c1-2-4-13-5-3-7-8(6-13)10(12)14-9(7)11/h2-6,11-12H2,1H3
- InChIKey RUDWASKCQMKNHV-UHFFFAOYSA-N
- SMILES C1(N)=C2CN(CCC)CCC2=C(N)S1
- CAS DataBase Reference 104632-26-0(CAS DataBase Reference)
- FDA UNII 83619PEU5T
- ATC code N04BC05
Safety
- HS Code :2934100090
- Hazardous Substances Data :104632-26-0(Hazardous Substances Data)
-
Symbol(GHS)

- Signal wordWarning
- Hazard statements H332-H317-H312-H412-H302
- Precautionary statements P264-P270-P301+P312-P330-P501-P261-P271-P304+P340-P312-P273-P501-P280-P302+P352-P312-P322-P363-P501-P261-P272-P280-P302+P352-P333+P313-P321-P363-P501
Pramipexole Chemical Properties,Usage,Production
- Chemical Properties Off-White Solid
- Originator Mirapex, Pharmacia and Upjohn, USA
- Uses partial/full D2S, D2L, D3, D4 receptor agonist
- Uses (S)-Pramipexole enantiomer. s disease (PD) and restless legs syndrome (RLS).[1] It is also sometimes used off-label as a treatment for cluster headache and to counteract the problems with sexual dysfunction experienced by some u sers of the selective serotonin reuptake inhibitor (SSRI) antidepressants.
- Uses (S)-Pramipexole enantiomer. A dopamine-D2-receptor agonist. Antiparkinsonian
- Definition ChEBI: A member of the class of benzothiazoles that is 4,5,6,7-tetrahydro-1,3-benzothiazole in which the hydrogens at the 2 and 6-pro-S-positions are substituted by amino and propylamino groups, respectively.
-
Manufacturing Process
75.5 g (0.5 mol) of 4-aminocyclohexanol hydrochloride and 74.0 g (0.5 mol) of phthalic acid anhydride are mixed with 65 g (0.5 mol) of ethyldiisopropyl amine and 1000 ml of toluene and boiled for 36 hours with a water separator. Then water is added, the toluene phase is separated off and the aqueous phase is extracted several times with chloroform. The organic phases are combined, dried and concentrated. The concentrated residue is recrystallised from isopropanol and 4-(phthalimido)-cyclohexanol was obtained. Yield: 95 g (77.8%). Melting point 175°-176°C.
95 g (0.388 mol) of 4-(phthalimido)-cyclohexanol are dissolved in 600 ml of chloroform and, after the addition of 450 ml of water and 120 ml of sulfuric acid, 90 g (0.3 mol) of potassium dichromate are added in batches. The internal temperature of the mixture is maintained at between 25° and 30°C by slight cooling. The mixture is stirred for a further 3 hours, then the chloroform phase is separated off and the mixture extracted twice more with chloroform. After drying and concentration of the extracts 82 g (86.9%) of 4(phthalimido)-cyclohexanone was obtained.
48.6 g (0.2 mol) of 4-(phthalimido)cyclohexanone are dissolved in glacial acetic acid, mixed with 36% of hydrobromic acid in glacial acetic acid and then 32 g (0.2 mol) of bromine in glacial acetic acid is added dropwise with cooling. The mixture is then concentrated by evaporation in vacuo and the residue is triturated several times with diethylether. The ether extracts are discarded and the residue is dissolved in of ethanol. After thiourea have been added the mixture is refluxed for 5 hours. It is then concentrated by evaporation, made alkaline with sodium hydroxide solution and extracted with chloroform. After drying and concentration of the extracts, the residue is purified by column chromatography on silica gel (eluant: chloroform/methanol = 1/1). The 2-amino-6-phthalimido-4,5,6,7-tetrahydro-benzthiazol was obtained. Melting point 244-246°C, dec. Yield: 30 g (50%).
9.5 g (31.7 mmol) of 2-amino-6-phthalimido-4,5,6,7-tetrahydro-benzthiazole are suspended in 100 ml of ethanol and, after the addition of 1.8 g (36 mmol) of hydrazine hydrate, refluxed for 2 hours. The mixture is then concentrated and purified by column chromatography on silica gel using methanol as eluant. The 2,6-diamino- 4,5,6,7-tetrahydro-benzthiazole was obtained.
To a solution of 2,6-diamino- 4,5,6,7-tetrahydro-benzthiazole in dimethylformamide are added n-propanal and the mixture is heated to 50°C for 1 hour. After cooling, the reaction solution is mixed with sodium borohydride and heated to 50°C for 30 min. The solvent is largely eliminated in vacuo. Whilst cooling with ice, the residue is mixed with water and 2 N hydrochloric acid until a pH of 1 is obtained. The aqueous solution is exwith ethylacetate and the organic phase discarded. The aqueous phase is mixed with potassium carbonate until an alkaline reaction is obtained and then extracted with ethyl acetate. The organic phase is dried and concentrated. The 2-amino-6-n-propylamino-4,5,6,7-tetrahydro-benzthiazole dihydrochloride crystallizes out when ethereal hydrochloric acid is added. Yield: 42%. Melting point: 286°-288°C. - Therapeutic Function Antiparkinsonian, Antipsychotic
- Biological Activity pramipexole is a dopamine agonist of the non-ergoline class indicated for treating parkinson's disease (pd) and restless legs syndrome (rls).pramipexole also possesses low/insignificant affinity (500-10,000 nm) for the 5-ht1a, 5-ht1b, 5-ht1d, and α2-adren
Pramipexole Preparation Products And Raw materials
Raw materials
Preparation Products
Global(360)Suppliers
-
Supplier:
Qingdao Yonghefeng Economic and Trade Co., Ltd.
- Tel:+86-13375559818<br/>+86-13375559818
- Email:cfy69@163.com
- Country:China
- ProdList:195
- Advantage:58
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8615531157085
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8808
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86 13288715578<br/>+8613288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12810
- Advantage:58
-
Supplier:
HangZhou RunYan Pharma Technology Co.,LTD.
- Tel: +8618882027439
- Email:sales2@runyanpharma.com
- Country:China
- ProdList:302
- Advantage:58
-
Supplier:
Shandong Xuhuang New material CoLTD
- Tel:+86-15095020839<br/>+86-17660422208
- Email:
- Country:China
- ProdList:726
- Advantage:58
-
Supplier:
Capot Chemical Co.,Ltd.
- Tel:+86-(0)57185586718<br/>+86-13336195806
- Email:sales@capot.com
- Country:China
- ProdList:29730
- Advantage:60
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21628
- Advantage:55
-
Supplier:
career henan chemical co
- Tel:+86-0371-86658258<br/>+8613203830695
- Email:sales@coreychem.com
- Country:China
- ProdList:29859
- Advantage:58
-
Supplier:
Jinan Jianfeng Chemical Co., Ltd
- Tel:+86-0531-88110457<br/>+86-15562555968
- Email:info@pharmachemm.com
- Country:China
- ProdList:334
- Advantage:58
-
Supplier:
Chengdu Feibo Pharm Technology Co., Ltd
- Tel:+86 028-84641798<br/>15982321820
- Email:abby@arkpharm.com
- Country:China
- ProdList:10922
- Advantage:58
Related articles
104632-26-0, PramipexoleRelated Search:
- Pramipexole EP Impurity E 6-Aminocaproic acid Glycine Isopropyl alcohol Isophorondiamine 2-(2-Aminothiazol-4-yl)glyoxylic acid Propyl butyrate 3-Aminopropyltriethoxysilane HYDRAZINE Lithium diisopropylamide Propyl gallate ALTRENOGEST Tetrapropylammonium hydroxide 2-Aminothiazole-4-acetic acid Tris(hydroxymethyl)aminomethane Pramipexole impurity 7 2-Aminophenol Citalopram N-Oxide
- Pharmaceuticals
- Intermediates & Fine Chemicals
- Inhibitors
- All Inhibitors
- Sulfur & Selenium Compounds
- Chiral Reagents
- API
- Mirapexin, Sifrol
- 杂质对照品
- 抑制剂
- 药靶配体
- FDA批准的配体
- 威德利现货找张军
- 主打产品
- 科研原料
- 标准品
- 对照品
- 化学试剂
- 化工中间体
- 原料药及中间体
- 化工试剂
- 医用原料
- 原料
- 化工原料药
- 对照品-杂质对照品
- 医用原料
- 医药原料
- 医药中间体
- 神经系统药物
- 神经信号
- 中间体
- 原料药
- 中枢神经系统用药
- 药物
- 抗癫痫药
- 碱基形式
- 小分子抑制剂
- C10H17N3S
- C10H21Cl2N3OS
- C10H18ClN3S
- 普拉克索,10 MM DMSO 溶液
- 普拉克索碱基/普拉克索N-1
- [(6S)-2-氨基-4,5,6,7-四氢-1,3-苯并噻唑-6-基]-CHEMICALBOOK丙基-胺
- 普拉克索 PRAMIPEXOLE
- 普拉克索碱(对照品)
- (S)-2-氨基-4,5,6,7-四氢-6-(丙氨基)苯并噻唑
- 森福罗
- 盐酸普拉克索杂质D对照品
- 米拉帕
- (S)-2-氨基-4,5,6,7-四氢-6-丙基氨苯噻唑
- (S)-N6-丙基-4,5,6,7-四氢苯并[D]噻唑-2,6-二胺
- 普拉克索,帕尔米斯
- 2-氨基-4,5,6,7-四氢-6-丙基氨苯噻唑普拉克索
- 普拉克素
- 普拉克索杂质06
- 普拉克索
- 2-氨基-4,5,6,7-四氢-6-丙基氨苯噻唑
- (S)-4,5,6,7-四氢-N^<6>^-丙基-2,6-苯并噻唑二胺