ChemicalBook > CAS DataBase List > Polychloroprene
Polychloroprene
Polychloroprene
- CAS No.9010-98-4
- Chemical Name:Polychloroprene
- CBNumber:CB0414643
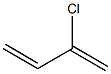
- Molecular Formula:C4H5Cl
- Formula Weight:88.5355
- MOL File:9010-98-4.mol
Polychloroprene Property
- Melting point >260 °C
- Density 1.23 g/mL at 25 °C(lit.)
- storage temp. -196°C
- form chunks
- color White to beige
- Viscosity 40Mooney(lit.)
- Dielectric constant 6.0(Ambient)
- InChI InChI=1S/C4H5Cl/c1-3-4(2)5/h3H,1-2H2
- InChIKey YACLQRRMGMJLJV-UHFFFAOYSA-N
- SMILES C=C(Cl)C=C
- Indirect Additives used in Food Contact Substances POLYCHLOROPRENE
- CAS DataBase Reference 9010-98-4(CAS DataBase Reference)
- EWG's Food Scores 1
- IARC 3 (Vol. 19, Sup 7) 1987
- EPA Substance Registry System Chloroprene polymer (9010-98-4)
- UNSPSC Code 12162002
- NACRES NA.23
Safety
- Safety Statements :24/25
- WGK Germany :3
- RTECS :EI9640000
- Hazardous Substances Data :9010-98-4(Hazardous Substances Data)
-
NFPA 704:
0 1 0
- Symbol(GHS)
- Signal word
- Hazard statements
- Precautionary statements
Polychloroprene Price
More Price(2)
- Brand: Sigma-Aldrich(India)
- Product number: 205400
- Product name : Polychloroprene
- Purity: 85% trans, 10% cis
- Packaging: 250G
- Price: ₹10678.2
- Updated: 2022/06/14
- Buy: Buy
- Brand: Sigma-Aldrich(India)
- Product number: 205397
- Product name : Polychloroprene
- Purity: 85% trans, 10% cis
- Packaging: 250G
- Price: ₹11754.9
- Updated: 2022/06/14
- Buy: Buy
Polychloroprene Chemical Properties,Usage,Production
- Description Neoprene, also known as polychloroprene and chloroprene rubber, is an elastomer produced by polymerization of chloroprene.
-
Chemical Properties
The appearance of neoprene is milky white, beige or light brown flakes or lumps. Its density is 1.23–1.25 g.cm–3. The
solubility parameter δ of neoprene is 9.2–9.41. Neoprene is soluble in toluene, xylene,
chloroform, and dichloroethane, and slightly soluble in acetone, methyl ethyl ketone,
ethyl acetate, cyclohexane but insoluble in n-hexane and solvent gasoline. Neoprene has good physical, chemical and mechanical properties. In comparison with natural rubber, it is much denser, more resistant to water and hydrocarbon
solvents, less permeable to many gases, and was more resistant to degradation by
oxygen, ozone, hydrogen chloride, hydrogen fluoride, and other chemicals. It has
certain flame retardance. The decomposition temperature is 230–260°C, and it can
be used for a long time at 80–100°C.
-
History
Polychloroprene was discovered in 1930 at E. I. DuPont de Nemours & Co. inWilmington Delaware. The discovery grew out of a need to develop a synthetic substitute for natural rubber. DuPont first marketed this first commercially successful synthetic elastomer as DuPrene in 1933. In response to new technology development that significantly improved the product and manufacturing process, the name was changed to Neoprene in 1936. The current commercially acceptable generic name for this class of chlorinated elastomers is CR or chloroprene rubber.
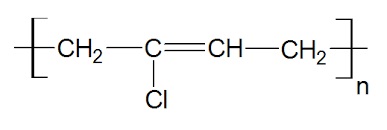
polychloroprene structure - Uses Polychloroprene is a (Solid) Mechanical rubber products, lining oil-loading hose and reaction equipment, adhesive cement, binder for rocket fuels, coatings for electric wiring, gaskets and seals. (Liquid) Specialty items made by dipping or electrophoresis from the latex. (Foam) Adhesive tape to replace metal fasteners for automotive accessories, seat cushions, carpet backing, sealant
-
Production Methods
Commercial polychloroprene rubber is manufactured by aqueous free-radical emulsion polymerization followed by isolation of the solid polymer by one of several processes: freeze roll isolation, drum drying , extruder isolation, precipitation and drying or spray drying. Isolation of powdered polychloroprene has been reviewed. Of the methods cited, freeze roll and drum drying isolation are commercially important.
The large-scale commercial manufacture of polychloroprene consists of eight or nine unit operations:
(1) Monomer solution makeup Water solution makeup
(2) Emulsification
(3) Polymerization
(4) Stripping of residual monomer
(5) Peptization for chloroprene–sulfur copolymers
(6) Freeze roll isolation Drum drying
(7) Drying of freeze-rolled film
(8) Roping
(9) Cutting and packaging (25kg) - Preparation Polychloroprene is made from one of two starting materials, acetylene or butadiene. Acetylene can be dimerized and then chloronated to form chloroprene. Alternatively, when adequate butadiene is available, this can be directly halogenated (eqs. 7 and 8). In either case, the chloroprene product can then be polymerized to polychloroprene. Essentially a butadiene elastomer with chlorine present in the backbone,the polymer exhibits excellent tensile strength and low hysteresis, much like natural rubber. Tensile strength properties up to 28 MPa are possible with the proper reinforcing system (see FILLERS). The polarity imparted by the chlorine atom improves the oil and solvent resistance approaching those of nitrile polymers. The polymer can be protected with para-phenylenediamine antiozonants to give ozone resistance, and heat aging is also good. As a result, chloroprene elastomers are used in a wide variety of applications needing a balance of such properties.
- Definition ChEBI: A macromolecule composed of repeating (2Z)-2-chlorobut-2-ene-1,4-diyl units.
- Hazard Questionable carcinogen.
-
Industrial uses
One of the first synthetic rubbers used commercially to the rubber industry, neoprene is a polymer of chloroprene, 2-chlorobutadiene- 1,3. In the manufacturing process, acetylene, the basic raw material, is dimerized to vinylacetylene and then hydrochlorinated to the chloroprene monomer.
Sulfur is used to vulcanize some types of neoprene, but most of the neoprenes are vulcanized by the addition of basic oxides such as magnesium oxide and zinc oxide. The cure proceeds through reaction of the metal oxide with the tertiary allylic chlorine that arises from the small amount of 1,2-polymerization that occurs. Other compounding and processing techniques follow similar procedures and use the same equipment as for natural rubber. One of the outstanding characteristics of neoprene is the good tensile strength without the addition of carbon black filler. This versatility makes them useful in many applications requiring oil, weather, abrasion, or electrical resistance or combinations of these properties, such as wire and cable, hose, belts, molded and extruded goods, soles and heels, and adhesives. - Materials Uses The most widely used contact adhesive is a solution of polychloroprene or modified polychloroprene in solvent blends of aromatic hydrocarbons, aliphatic hydrocarbons, esters, or ketones, for example, toluene–hexane–acetone. Viscosity, dry time needed before bonding, bond strength, and price are affected by the solvent. Using various combinations of the isomeric forms of polymerized 2- chlorobutadiene permits a fine-tuning of the crystallization rate of the dissolved polymer as the solvent evaporates. The polychloroprene may also be modified by the incorporation of methacrylic acid or mercaptans. Metal oxides (MgO and ZnO) that scavenge acids are often part of polychloroprene adhesives and also may act as cross-linking agents. Oxygen scavengers such as butylated hydroxytoluene (BHT) [128-37-0] or naphthylamines [25168-10-9] are added to prevent dehydrochlorination. To build initial handling strength, the solvent-based polychloroprene contact adhesives may be modified with alkyl phenolics, terpene phenolics, or phenolic-modified rosin esters, the first of these being the most effective and least deleterious. Chlorinated rubbers are sometimes added to these adhesives to improve their adhesion to plasticized PVC and other plastics. Added just before adhesive application, isocyanates are useful in modification of polychloroprene contact adhesives, reacting perhaps through hydrolysis of the pendant allylic groups present from the small number of 1,2 isomeric segments. The remainder of the solvent-based contact adhesives are comprised of polyurethane, SBR, styrene–butadiene–styrene block polymers, butadiene–acrylonitrile rubber, natural rubber, or various acrylic or vinyl resins in suitable solvents.
- Advantages By proper raw material selection and formulation of these polymers, the compounder can achieve optimum performance for a given end-use. Initially developed for resistance to oils and solvents it may resist various organic chemicals including mineral oils, gasoline, and some aromatic or halogenated solvents. It also exhibits good chemical resistance to aging and attack by ozone, and good resistance to UV irradiation (e.g., exposure to sunlight), until moderately elevated temperatures. Moreover, it has outstanding resistance to damage caused by flexing and twisting, an elevated toughness, and it resists burning but its electrical properties are inferior to that of natural rubber. Therefore, neoprene (polychloroprene) is noted for a unique combination of properties which has led to its use in thousands of applications throughout industry.
Polychloroprene Preparation Products And Raw materials
Raw materials
Preparation Products
Global(163)Suppliers
-
Supplier:
Hebei Chuanghai Biotechnology Co., Ltd
- Tel: +8617732866630
- Email:abby@chuanghaibio.com
- Country:China
- ProdList:8773
- Advantage:58
-
Supplier:
Hebei Mujin Biotechnology Co.,Ltd
- Tel:+86-13288715578
- Email:sales@hbmojin.com
- Country:China
- ProdList:12648
- Advantage:58
-
Supplier:
Henan Bao Enluo International TradeCo.,LTD
- Tel:+86-17331933971
- Email:deasea125996@gmail.com
- Country:China
- ProdList:2472
- Advantage:58
-
Supplier:
Hebei Kingfiner Technology Development Co.Ltd
- Tel:+86-15532196582<br/>+86-15373005021
- Email:lisa@kingfinertech.com
- Country:China
- ProdList:299
- Advantage:58
-
Supplier:
Yujiang Chemical (Shandong) Co.,Ltd.
- Tel: +8617736087130
- Email:catherine@yjchem.com.cn
- Country:China
- ProdList:994
- Advantage:58
-
Supplier:
Hebei Zhuanglai Chemical Trading Co Ltd
- Tel:+86-16264648883
- Email:niki@zlchemi.com
- Country:China
- ProdList:7245
- Advantage:58
-
Supplier:
Henan Tianfu Chemical Co.,Ltd.
- Tel:+86-0371-55170693<br/>+86-19937530512
- Email:info@tianfuchem.com
- Country:China
- ProdList:21622
- Advantage:55
-
Supplier:
.GZ HONESTCHEM CO.,LTD
- Tel:+86-15013270415
- Email:honestchemical@foxmail.com
- Country:China
- ProdList:246
- Advantage:58
-
Supplier:
SHANDONG ZHI SHANG CHEMICAL CO.LTD
- Tel: +86 18953170293
- Email:sales@sdzschem.com
- Country:China
- ProdList:2930
- Advantage:58
-
Supplier:
Hubei Jusheng Technology Co.,Ltd.
- Tel:18871490254
- Email:linda@hubeijusheng.com
- Country:CHINA
- ProdList:28172
- Advantage:58
Related articles
9010-98-4, PolychloropreneRelated Search:
- Hexachloro-1,3-butadiene Methylene dithiocyanate CIS-BUTENEDIOIC ANHYDRIDE Polybutene Cyanuric chloride Epichlorohydrin FLUOROLUBE GREASE, GR-362 POLYBUTADIENE DIACRYLATE POLY(ETHYLENE) Polyvinyl chloride 1,3-Butadiene SILICONE RUBBER EPDM RUBBER Neoprene adhesive Chloroacetic acid Hydroyl-Terminated Polybutadiene CSM Difluorochloromethane
- Polymer Science
- Hydrophobic Polymers
- Dienes
- Polymers
- Polychloroprene
- Polymer Science
- Materials Science
- Hydrophobic Polymers
- 细胞系-人细胞系
- 日用化工
- 天然植物胶体&发酵多糖
- 氯丁胶乳
- ATCC细胞-ATCC细胞系
- 细胞-细胞系
- 原料药
- 乳胶
- 合成材料中间体
- 固体类
- 有机原料
- 精细化工品
- 化工原料
- 增稠剂
- 化工原料
- C4H5CLX
- -CH2CHCClCH2n
- C4H5Cln
- -CH2CClCHCH2n
- GRM人胰腺癌细胞
- 小鼠乳腺癌细胞GR-M
- 氯丁橡胶 沙蒿胶
- 10%顺式,85%反式
- 氯丁橡胶,门尼粘度40,40 WT%氯
- 氯丁胶板
- 2-氯-1,3-丁二烯的均聚物
- 阳离子氯丁胶乳
- 羧基氯丁胶乳
- 氯丁橡胶
- 氯丁胶乳(LDR-401型)
- 羧基氯丁胶乳(LSR-511型)
- 阳离子氯丁胶乳(LDR-501Y型)
- 聚氯丁烯
- 2-氯-1,3-丁二烯均聚物
- 氯丁橡胶(LDJ-120型)
- 氯丁橡胶(LDJ-230型)
- 氯丁胶乳(LDR-503型)
- 复合调节非污染氯丁橡胶(LDJ-320F型)
- 氯丁胶乳(LDR-403型)
- 氯丁橡胶(LDJ-240型)
- CR244型氯丁橡胶
- 氯丁胶乳
- 氯丁二烯橡胶
- 聚氯丁烯,氯丁橡胶
- 聚氯丁二烯
- 9010-98-4
- Polychloroprene,Mooney viscosity 40,40 wt% Chlorine
- Nicotinamide riboside chloride 23111-00-4
- Unvulcanised polychloroprene
- Polychloroprene, Unvulcanised