Description
Bromothymol blue is a pH indicator for weak acids and bases. In neutral solution, it appears bluish-green. At pH above 7.6 it appears blue, and at pH below 6.0 it appears yellow.
Bromothymol blue (BTB) is an anionic dye. It can be protonated or deprotonated to generate yellow or blue. BTB helps to make ion-pair complex, that is used to identify several pharmaceutical compounds with the help of extractive spectrophotometric methods.
Chemical Properties
Pale purple crystalline powder
Uses
Bromothymol blue is mostly used in measuring substances that would have relatively low acidic or basic levels (near a neutral pH). It's often used in pools, fish tanks, or measuring the presence of carbonic acid in a liquid.
Bromothymol Blue is a sulfonated hydroxyquinone in acidic solutions. In alkaline solutions, it is present as a dianion that is blue with a larger conjugated system than the yellow hydroxyquinone. Both forms are lipophilic. It is also known as bromthymol blue or dibromothymolsulfonphthalein.
Uses
Bromothymol blue is a chemical indicator for weak acids and bases.
Uses
Bromothymol Blue is a pH indicator used for measuring pH around 7 in pools and fish tanks. It operates in the pH range 6.0 to 7.6 due to the presence of an electron withdrawing and donating groups like bromine atoms and alkyl substitutents respectively. In the laboratory, it is used as a biological slide stain as well as used in obstetrics for detecting premature rupture of membranes. Further, it is used for the observation of photosynthetic activities and a respiratory indicator. In addition to this, it is used to define cell walls or nuclei under the microscope.
Definition
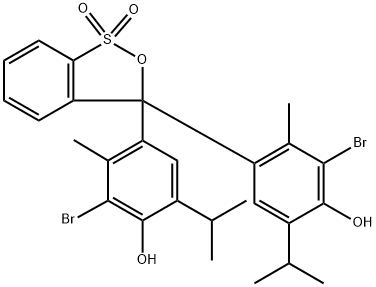
ChEBI: A member of the class of 2,1-benzoxathioles that is 2,1-benzoxathiole 1,1-dioxide in which both of the hydrogens at position 3 have been substituted by 3-bromo-4-hydroxy-5-isopropyl-2-methylphenyl groups.
Application
Bromothymol blue has various applications in biology and medicine. It is suitable for demonstrating fungal hyphae within plant roots. It is used as a vital stain to trace the movement of fluids from the lymph. It is added to an agar gel microbial culture medium for enumeration of Bacillus cereus. Bromothymol blue is also used as a pH indicator due to its precise color transition properties at neutrality.
Suitable for use:
In preparations of pH indicator solutions.
In phospholipid staining on tissues and cells prepared on microscopy slides.
Preparation
Bromothymol blue is synthesized by adding elemental bromine to the thymol blue in a solution of glacial acetic acid.
To prepare a solution that is used as a pH indicator, we should dissolve 0.10 g in an 8.0 cm3 N/50 NaOH and then dilute it with water to 250 cm3. To prepare a solution used as an indicator in volumetric work, we should dissolve 0.1 g in the 100 cm3 of 50% (v/v) ethanol.
General Description
Bromothymol blue is a pH indicator which is sparingly soluble in water, benzene but is soluble in ethanol. It is insoluble in petroleum ether. It has its major uses in Sol-gel matrix, fuel cells, optical sensors, combustion gas detection system. It is considered as a toxic reagent.
Purification Methods
Dissolved the dye in aqueous 5% NaHCO3 solution and precipitate it from the hot solution by dropwise addition of aqueous HCl. Repeat this until the extinction at max 420nm does not increase. It is an indicator: aqueous solutions are yellow at pH 6.0, and blue at pH 7.6. [Beilstein 19/3 V 461.]