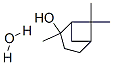
Chemical Properties
White or colorless crystals. M.P. 148℃. Insoluble in water, soluble in alcohol and oils.
Originator
Sobrepin,Corvi,Italy,1970
Uses
Pinol hydrate could find use in detergent perfumes, fragrances for household products, etc.
Preparation
Pinol hydrate is produced by hydrolytic autoxidation of alpha-pinene. It is also formed by heating of Pinol, or by treatment of Pinene with Lead tetra-acetate.
Definition
ChEBI: Sobrerol is a p-menthane monoterpenoid.
Manufacturing Process
To 19 l of well-agitated distilled water plus 18 g of ditertiary-butyl-p-cresol
was added 19.84 kg (130 mols) of pure α-pinene oxide that was about half
racemic, half d-form. The temperature was maintained at 30°C to 50°C, first
with ice bath cooling and then with tap water cooling. The addition of the
pinene oxide required 1,5 hours. After the addition was complete and the
exothermic reaction was about over, the mixture was stirred for 2,5 hours at
about 30°C, and then centrifuged to separate the crude sobrerol from the
liquid phase consisting of oil and water.
The crude sobrerol was washed with naphtha and then air dried to yield 14.81
kg (87.5 mols) of pure sobrerol, [α]D
25 -77.0°. It was found that 1 liter of the
aqueous phase from the reaction contained 22 g of sobrerol, so, therefore, the
entire aqueous phase contained 0.42 kg (2.5 mols) of sobrerol.
Therapeutic Function
Mucolytic