Description
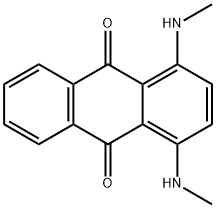
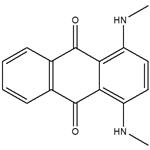
Disperse blue 14 is also known as transparent blue GP. Its chemical name is 1,4-bis(methylamino)anthraquinone. It is a blue-black powder, insoluble in water, soluble in methanol, ethanol, glacial acetic acid, nitrobenzene, pyridine and toluene. It is reddish brown in concentrated sulfuric acid.

Uses
1,4-Bis(methylamino)anthraquinone can be useful in freshwater production by solar desalination of seawater using two-ply dye modified membrane system. Disperse Blue 14 [1,4-bis(methylamino) anthracene-9,10-dione] has been used in dye formulations to make violet smokes and fireworks in addition to color fabrics and lubricants[1].
Preparation
1,4-Dihydroxyanthracene-9,10-dione?by under pressure,?With aqueous Methanamine?and sodium hydrosulfite or zinc processing. Leuco sulfate nitrobenzene oxidation or thermal
Application
Disperse Blue 14 is an important organic dye for the dyeing of polyester, vinegar and polyamide fibres and for transfer printing. It is also suitable for the manufacture of firework colouring components. It can also be used for surface colouring and plastics etc.
Toxicology
Disperse Blue 14 has been found to cause behavioral and developmental effects in zebrafish embryos. Disperse Blue 14 had a greater impact than Solvent Red 169 and Disperse Red 9 on the endpoints tested. Disperse Blue 14 had a LOEL of 261.5 μM for mortality, caused yolk sac edema and hypoactivity in the dark phase at all concentrations, and had a LOEL of 133 μM for gene expression, with 314 μM affecting the most DEGs (2454 DEG vs. 18–174 for other treatment groups) at the 133–314 μM range tested. Gene expression analysis indicated that the dye caused toxic effects at all concentrations but few effects on neurodevelopmental processes. The dye causes developmental and behavioral effects at much lower concentrations, with a LOEL of 20.2 μM for developmental effects and hyperactivity in light and 0.66 μM for hypoactivity in the dark[1].
Purification Methods
Purify the anthraquinone by thin-layer chromatography on silica gel plates, using toluene/acetone (3:1) as eluent. The main band is scraped off and extracted with MeOH. The solvent is evaporated and the dye is dried in a drying pistol [Land et al. J Chem Soc, Faraday Trans 1 72 2091 1976]. It crystallises from n-butanol with m 221-222o and has max 539 and 644nm (EtOH). [Beilstein 14 H 198, 14 III 440, 14 IV 459.]
Properties and Applications
bright blue. Blue powder. Soluble in acetone, glacial acetic acid, the difficulties, pyridine and toluene. The strong sulfuric acid to red light brown. Used for polyester, vinegar and polyamide fiber dyeing, also can be used to transfer printing. And is fit for manufacturing fireworks fireworks hair color components. Can also be used in such of surface colouring and plastic.
|
Standard(Polyamide)
|
Ironing Fastness
|
Light Fastness
|
Persperation Fastness
|
Washing Fastness
|
|
Fading
|
Stain
|
Fading
|
Stain
|
Fading
|
Stain
|
|
ISO
|
4
|
2-3
|
4-5
|
4-5
|
2-3
|
4
|
3-4
|
References
[1] Edward J Perkins. “Developmental, Behavioral and Transcriptomic Changes in Zebrafish Embryos after Smoke Dye Exposure.” Toxics (2022).