References
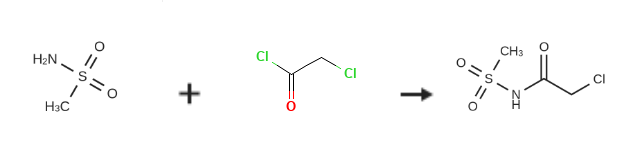
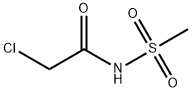
N-(chloracetyl)methanesulfonamide is prepared by the reaction of methanesulfonamide and chloroacetyl chloride. The specific synthesis steps are as follows:
In a 2-liter reaction flask, 1 liter of ethyl acetate and 66 grams of methylsulfonamide were added, and 109 grams of chloroacetyl chloride was gradually added; the temperature was gradually increased to 65C for 12 hours until the end of the reaction.The reaction solution gradually cooled to 0 degree, and a large amount of white solid precipitated; it was filtered and dried to obtain 112 g of solid SLP-10b (X=Cl).Yield: 94%.H NMR (400MHz, CDCl3): delta 4.02 (s, 2H), 3.28s, 3H)ESI/MS+(m/z):171.