Description
LGD-4033 is a Selective Androgen Receptor Modulator (SARM). SARMs are a class of non-steroidal anabolic agents that bind androgen receptors with high affinity. LGD-4033 has been shown to provide benefits similar to traditional anabolic agents, (e.g., increased muscle mass, fat loss, and improved bone density) but with far fewer side effects (e.g., oily skin, acne, over-conversion to estrogen, etc.). Both men and women may also appreciate improved libido. All of these benefits are achieved without increasing androgen levels. Its anabolic effects are roughly the same or greater than testosterone, even at low doses. Some prescribers will recommend a trial of SARMs for low testosterone levels prior to utilizing testosterone replacement therapy.
Uses
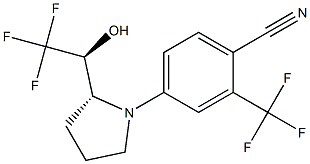
Ligandrol, also known as LGD-4033, is a nonsteroidal oral drug used for the treatment of muscle wasting and osteoporosis in healthy young men.
Biological Activity
LGD-4033 is a potent nonsteroidal selective androgen receptor modulator (SARM) that binds to the androgen receptor with a Ki value of 1 nM. In animal models, LGD-4033 displays robust selectivity for muscle versus prostate tissues, anabolic activity in the muscle, and anti-resorptive and anabolic activity in bone.
LGD 4033 has been abused as a performance-enhancing drug and the identification of its in vitro metabolites has been described.
This product is intended for research and forensic applications.
Mechanism of action
LGD-4033 works by binding to androgen receptors in muscle and bone tissue and stimulating protein synthesis, thus helping to build muscle and strength, increase bone density, reduce inflammation, and promote tissue healing. In addition, it can also help improve body composition by reducing fat mass while preserving lean muscle mass.
Pharmacokinetics
LGD-4033 functions by enlarging the androgen receptors, selectively. Anabolic effects on muscles and bones occur rather than negatively altering the prostate or glands, which could be caused through the use of steroids. LGD-4033 has recently been engaged in a study with volunteers called Phase 1 Multiple Ascending Dose. It was random,double blind testing phase in which placebo was used.The aim was to verify that the usage of LGD-4033 is safe and easy to use with a dosage not exceeding 22 mg daily.
Side effects
When taken in appropriate doses, most people experience minimal side effects, if any at all. Some users may experience mild headaches, nausea, or lightheadedness, but these are generally mild and go away quickly.
Safety
LGD-4033 was safe, had favorable pharmacokinetic profile, and increased
lean body mass even during this short period without change in
prostate-specific antigen. Longer randomized trials should evaluate its
efficacy in improving physical function and health outcomes in select
populations.
Synthesis
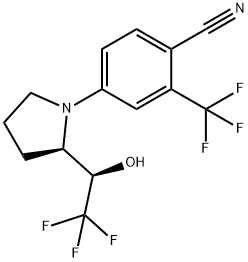
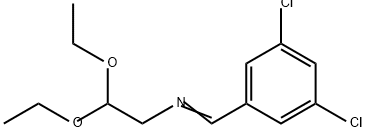
11.20 g Aldehyde (4-(2-(formylpyrrolidin-1-yl)-2-(trifluoromethyl)benzonitril )was dissolved in 300 ml dry tetrahydrofuran and 8.36 g CsF was added
directly as a solid to the reaction mixture. It was cooled via ice bath to 0°C and stirred for 15
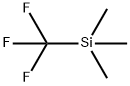
min. Then 25.00 g trimethyl(trifluoromethyl)silane was added via syringe over a period of 40
min. The reaction is stirred for 15 h coming from 0°C to room temperature over this time
interval. The reaction solution shifts color from nearly colorless to dark brown.
For stopping the reaction, it was diluted with 100 ml saturated ammonium chloride solution and
then evaporated. The resulting emulsion was extracted several times with ethyl acetate and the
combined phases dried over magnesium sulfate. After evaporation of all volatiles, the
intermediate silyl-ether can be obtained as red-brown oil.
This intermediate was converted to the final product LGD-4033 without any further purification.
The intermediate was diluted with 400 ml tetrahydrofuran and cooled to 0°C. A cooled
potassium hydroxide solution (4.76 g in 400 ml water) was mixed with the diluted oil and stirred
at 0°C for 2h. After quenching with 300 ml water, the organic solvent was removed via
evaporation from the mixture. The remaining brown oil was extracted with dichloromethane
and dried over magnesium sulfate, to yield a reddish-brown oil.
This crude product was chromatographed on silica gel by using hexane-ethyl acetat (8:2) as an
eluent. During purification, different fractions with several product isomers can be collected .
The isolated products LGD-4033 were obtained as 8.50 g yellowish oils (61%).