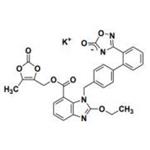
Uses
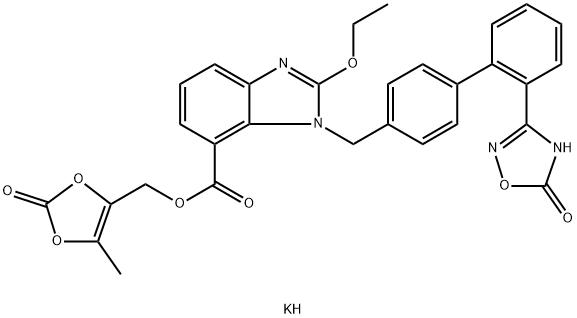
Azilsartan Kamedoxomil is an angiotensin II receptor blocker.
Definition
ChEBI: Azilsartan kamedoxomil is an organic potassium salt that is the monopotassium salt of azilsartan medoxomil. A prodrug for azilsartan, it is used for treatment of hypertension. It has a role as a prodrug, an antihypertensive agent and an angiotensin receptor antagonist. It contains an azilsartan medoxomil(1-).
Clinical Use
Azilsartan kamedoxomil, developed by Takeda Pharmaceuticals,
was approved for the treatment of hypertension and launched in
the U.S. under the brand name Edarbi. Edarbi is a prodrug that
undergoes rapid hydrolysis to liberate azilsartan, the active ingredient
(TAK-536, 39). As the 8th angiotensin receptor
blocker (ARB) to enter the world market, azilsartan kamedoxomil can function as monotherapy or in combination with other antihypertensive
agents. In several clinical studies, monotherapeutic azilsartan
kamedoxomil showed superior antihypertensive activity and a favorable safety/tolerability profile in patients compared
with other established therapeutics, including valsartan, olmesartan
medoxomil, candesartan, and telmisartan.In late 2011,Takeda announced that FDA also approved the fixed-dose combination
tablet of azilsartan kamedoxomil with chlorthalidone under
the trade name of Edarbyclor.
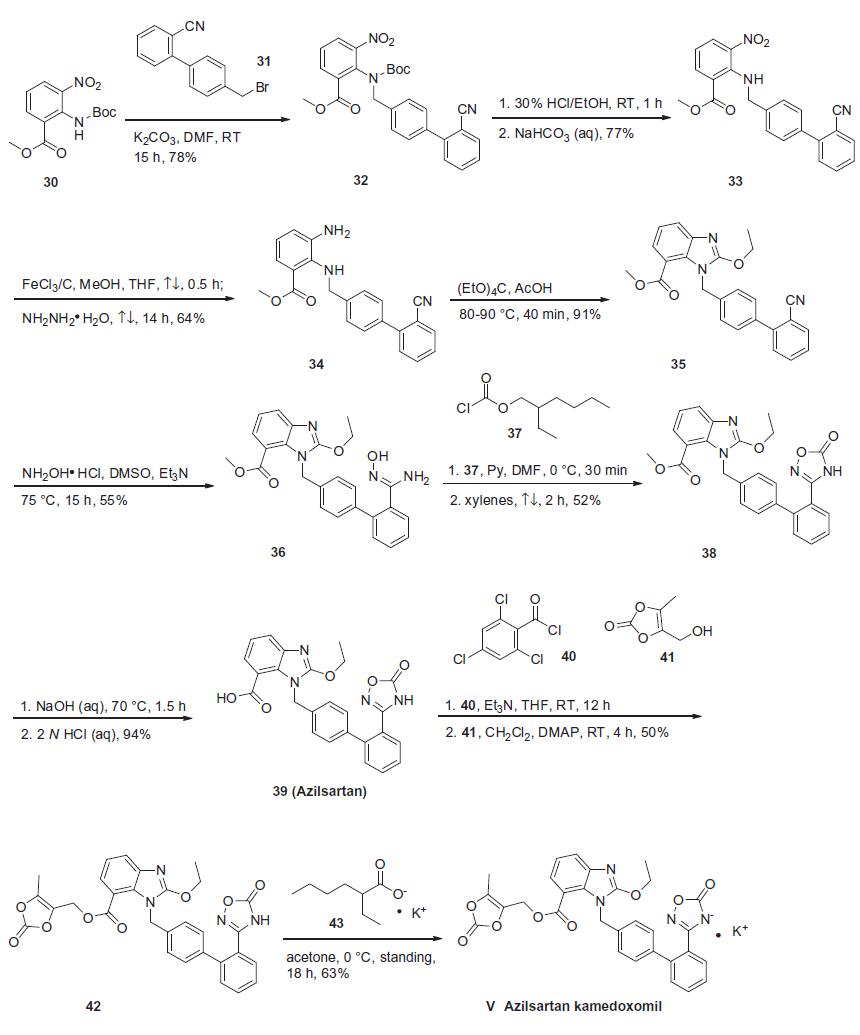
Synthesis
Based on the synthesis of azilsartan, the process-scale approach to azilsartan kamedoxomil is
described in the scheme. The synthesis started with commercially
available methyl 2-[(tert-butoxycarbonyl)amino]-3-nitrobenzoate
(30), which can also be prepared by several different routes.41,42
Alkylation of 30 with diaryl bromide 31 gave benzylamine 32 in
78% yield, which was followed by deprotection with 30% ethanolic
HCl and alkalinization to produce amine 33 in 77% yield. The nitro
group within 33 was reduced with hydrazine hydrate and a catalytic
amount of ferric chloride to afford 2,3-diaminobenzoate 34
in 64% yield. Ring formation was achieved by treatment of 34 with
tetraethoxymethane and acetic acid to produce benzimidazole 35
in 91% yield.37 The addition of hydroxylamine to the cyano group
of 35 provided amidoxime 36 in 55% yield, which underwent
immediate cyclization upon treatment with 2-ethylhexyl chloroformate
37 in refluxing xylenes to give oxadiazolone 38 in 52%
yield. Hydrolysis of 38 gave azilsartan (39, TAK-536) in 94%
yield. In the presence of perchlorobenzoyl chloride 40 and triethylamine,
carboxylic acid 39 was converted to the mixed acid
anhydride intermediate, which when condensed with alcohol 41
furnished benzoate 42 in 50% yield. Salt preparation of 42 was
accomplished with potassium 2-ethylhexyl carboxylate 43 affording
azilsartan kamedoxomil (V) in 63% yield.