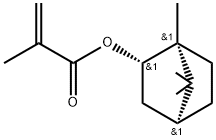
Description
Isobornyl methacrylate (IBOMA) is an acrylate monomer with a bridging ring structure. It can be used in polymerisation reactions to make other coatings or plastic products. Advantages of IBOMA and its polymers include: high viscosity, good compatibility, low volatility, low toxicity, UV resistance, water resistance, chemical resistance, and low adverse biological effects.
Chemical Properties
CLEAR COLORLESS TO YELLOW LIQUID
Characteristics
Isobornyl methacrylate is a colorless transparent liquid. It is a monomer that unifies hardness and flexibility. Due to its molecular structure, its polymer has excellent high gloss, sharp image, scratch resistance, medium resistance and weather resistance, and Its hygroscopicity is significantly lower than that of MMA (methyl methacrylate). In addition, IBOMA-added acrylic resin has good compatibility with polyester, alkyd and many volatile paint film-forming substances.
Uses
Isobornyl methacrylate is a versatile hydrophobic monomer with a good combination of hardness and flexibility that can improve the chemical and water resistance of polymer systems.
Application
Suitable for manufacturing acrylic resins with high glass transition temperatures (Tg).
Used as a thickening agent, base material, reactive diluent, and curing agent in printing ink formulations, solvent-based high-solid content transparent coatings, and curing coatings.
Suitable for producing decorative protective coatings for PET, PE, and PP soft plastic films, as well as engineering plastic components such as PE, PP, and PC.
Used as a plasticizer in the plastics industry.
Used as a viscosity improver in dental resin.
Preparation
Isobornyl methacrylate was synthesized by direct esterification of camphene and methacrylic acid.
General Description
Isobornyl methacrylate is a reactive solvent with low vapor pressure. It facilitates the free radical polymerization by forming a polymer with high glass transition temperature (T
g).
Flammability and Explosibility
Not classified
Synthesis
Isobornyl methacrylate is obtained by reacting (1S,2S,4S)-(+)-isoborneol and 4-methoxyphenol.Dissolve5g (1S,2S,4S)-(+)-isoborneol in 15 mL toluene. Add 4 mg (0.032 mmol) 4-methoxyphenol to the solution. After the addition of 0.4 g (3.24 mmol)4-dimethylamino pyridine, add a solution of 10 g (64.8 mmol) methacrylic anhydride (MAA) in 10 mL toluene dropwise to the mixture. Heat the reaction mixture to 80 °C. Stir the reaction mixture for 16 hours. After cooling down to room temperature, wash the mixture with 50 mL 1M sodium bicarbonate solution four times. Wash the organic phase with 50 mL brine two times. Separate the organic phase. Remove the toluene by rotary evaporation followed by a fractional distillation under reduced pressure.
1H NMR (CDCl3, 300 MHz): δ(ppm) = 6.06 (dq, 1H, 1); 5.51 (dq, 1H, 2); 4.71 (m ,1H, 4); 1.93 (dd, 3H, 3); 1.87-1.5 (m, 5H, 6,7,8); 1.23-1.04 (m, 2H, 5); 1.012 (s, 3H, 9);0.86 (s, 3H, 10); 0.85 (s, 3H, 11). IR (film): ν(cm-1) = 2954 (CH2), 2879 (CH2), 1715 (C=O) 1454 (CH2), 1157 and 1053 (C=O).
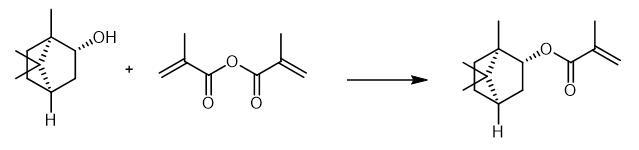
Fig The synthetic method of Isobornyl methacrylate