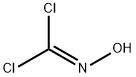
dichloroformoxine
- Product Namedichloroformoxine
- CAS1794-86-1
- CBNumberCB91363206
- MFCHCl2NO
- MW113.93
- MDL NumberMFCD09970438
- MOL File1794-86-1.mol
Chemical Properties
| Melting point | 39-40 °C |
| Boiling point | 129 °C |
| Density | 1.66±0.1 g/cm3(Predicted) |
| pka | 7.70±0.11(Predicted) |
| FDA UNII | G45S3149SQ |
| EPA Substance Registry System | Carbonimidic dichloride, hydroxy- (1794-86-1) |
Safety
| Hazardous Substances Data | 1794-86-1(Hazardous Substances Data) |
| Toxicity | It is absorbed through the skin, and contact with the material causes extreme pain immediately, much like lewisite, and tissue effects occur quickly. Irritation begins to occur in about 12 s at a dose of 0.2 mg-min/m3. When the agent has been in contact with the skin for 1 min or more, the irritation becomes unbearable at a dose of 3 mg-min/m3. It is also irritating to the respiratory system and causes the same types of eye damage as lewisite. Phosgene oxime remains persistent in the soil for about 2 h and is considered nonpersistent on other surfaces. The agent is known to be corrosive to most metals. There is no known antidote for phosgene oxime, and exposures should be treated symptomatically after decontamination, which should occur immediately. |