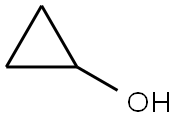
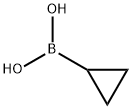
| Supplier |
Tel |
Email |
Country |
ProdList |
Advantage |
|
|
+86-(0)57185586718
+86-13336195806 |
sales@capot.com |
China |
29685 |
60 |
|
|
+86-0371-55170693
+86-19937530512 |
info@tianfuchem.com |
China |
21632 |
55 |
|
|
+86-0371-86658258
+8613203830695 |
sales@coreychem.com |
China |
29868 |
58 |
|
|
+8615866703830 |
figo.gao@foxmail.com |
China |
8497 |
58 |
|
|
+1-858-6993322 |
info@accelachem.com |
United States |
18568 |
58 |
|
|
+86-023-6139-8061
+86-86-13650506873 |
sales@chemdad.com |
China |
39894 |
58 |
|
|
28-85305008 |
|
CHINA |
964 |
58 |
|
|
+86-0551-65418671
+8618949823763 |
sales@tnjchem.com |
China |
34563 |
58 |
|
|
+86-0571-87635730
+8615858229168 |
chem1022@hengdian-group.com |
China |
1478 |
58 |
|
|
+86-27-87465837
+8618971612321 |
info@finetechnology-ind.com |
China |
9639 |
58 |