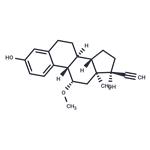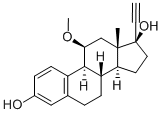
Moxestrol
- Product NameMoxestrol
- CAS34816-55-2
- CBNumberCB91179243
- MFC21H26O3
- MW326.43
- MDL NumberMFCD00198990
- MOL File34816-55-2.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 280° |
| alpha | D20 +29° (c = 0.6 in ethanol) |
| Boiling point | 404.46°C (rough estimate) |
| Density | 1.1348 (rough estimate) |
| refractive index | 1.4700 (estimate) |
| pka | 10.16±0.70(Predicted) |
| FDA UNII | 6923NT44OW |