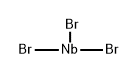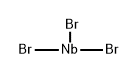Chemical Properties
Black, with varying air sensitivity, depending on preparative conditions. Almost completely resistant to H2O and dilute acids. Decomposed by concentrated H2SO4 and HNO3. Insoluble in organic solvents. It can be sublimed in a high vacuum (10-4 mm) at about 400°C. Thermal decomposition into NbBr5 and Nb begins at 900°C. The TaBr3 and the TaBr2 can be obtained in the same manner as NbBr3, starting from TaBr5 and H2 at 700°C; however, the purity and the yield are lower.
Production Methods
Containing boats with the niobium metal, it is dried in a stream of very pure N2 at 200°C. The furnace is then heated to 450°C, and dry Br2 vapor is introduced in an N2 stream. The nascent NbBr5 condenses at a. After complete bromination; the bromide is sublimed in a pure N2 stream at 270°C, passing through glass wool plug b and the constriction c into section d. Then, a stream of high-purity H2 is introduced, and NbBr5 is allowed to sublime slowly into tube section e, keeping it at 500° using furnace o2. Niobium bromide deposits on the tube wall as a shiny black crust and a black cone on the cold finger. The tube is opened, and the product is removed under a protective N3 blanket. The outer crusts are not air-sensitive and are insoluble in H3O. On the other hand, the cones deposited in the inner part of the apparatus decompose rapidly in the presence of moist air.