Industrial uses
Fatty acids of the formula C
nH
2n+1 are saturated. Unsaturated fatty acids have a formula
of C
nH
2n-1. Typical examples of saturated fatty acids are stearic acid,
C
17H
35COOH, and palmitic acid, C
15H
31COOH. The example for an unsaturated fatty acid
is oleic acid. From the flotation point of view, unsaturated fatty acids are much more
important than saturated fatty acids. Unsaturated fatty acids are more selective than saturated fatty acids.
Synthesis
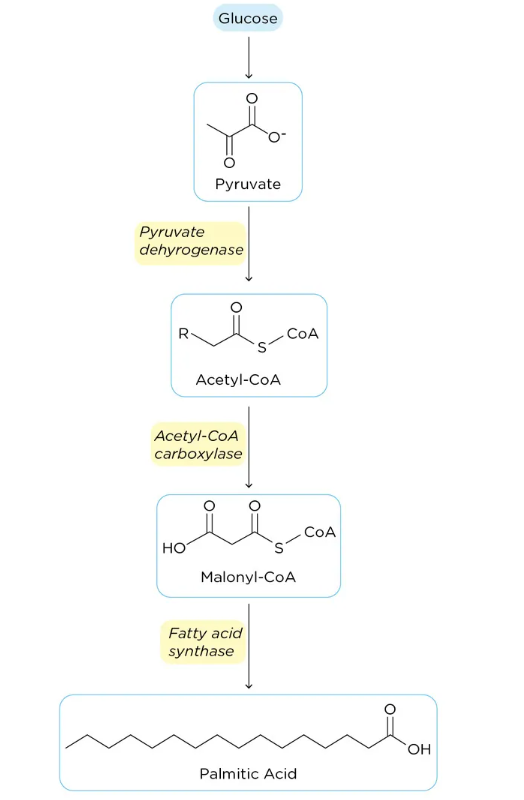
Fatty Acid synthesis occurs in the cytoplasm and endoplasmic reticulum. It is synthesised from acetyl-CoA in seven chain-extension steps, adding two carbons at a time to produce a 16-carbon fatty acid called palmitic acid; most of the synthesised fatty acid is esterified to triglycerides for storage. The synthesis pathway is mainly through the following steps:
(1) Source and transport of acetyl-CoA: Acetyl-CoA is converted from pyruvate during glycolysis. It is transported from the mitochondria to the cytoplasm in the form of citrate using.
(2) ATP citrate lyase in the cytoplasm converts citrate into acetyl-CoA and oxaloacetate (which returns to the mitochondria).
(3) Acetyl-CoA in the cytoplasm is carboxylated to malonyl-CoA (three carbon atoms) in an enzymatic reaction.
(4) Fatty acid synthase, a multi-enzyme complex that mediates chain elongation, makes use of malonyl-CoA as a substrate and combines it with another acetyl-CoA molecule to form a 4-carbon fatty acid (one carbon is released as COz).
(5) The addition of the two carbons is repeated seven times in a similar process to produce a 16-carbon fatty acid called palmitate. This process must be carried out in the presence of NADPH, which acts as a reducing agent. Palmitate can then be further processed, such as using esterification.