Chemical Properties
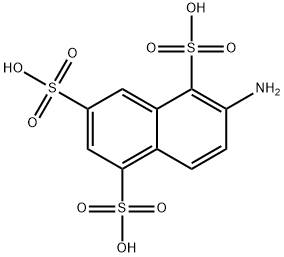
2-Aminonaphthalene-1,5,7-trisulfonic acid. (128) (6-aminonaphthalene1,3,5-trisulfonic acid), C10H9NO9S3, Mr 383.36, and its salts are readily soluble in water. Alkali fusion (65 % NaOH, 220℃) forms 2-amino-5- hydroxynaphthalene-1,7-disulfonic acid. Desulfonation to give 2-aminonaphthalene-5,7-disulfonic acid occurs on heating with dilute sulfuric acid. Diazotization and treatment with sodium carbonate yield the diazo oxide of 2-diazo-1- hydroxynaphthalene-5,7-disulfonic acid.
Production Methods
Vigorous sulfonation of Tobias acid to produce 2-aminonaphthalene-5,7-disulfonic acid is described. The intermediate 128 is obtained by quenching the sulfonation mass and liming out carefully to avoid desulfonation. The calcium salt solution is converted to the sodium salt with sodium carbonate and used as a total liquor for conversion to 2-amino-5-hydroxynaphthalene-1,7-disulfonic acid.