Description
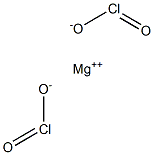
Magnesium chlorite has the molecular formula of
Mg(ClO2)2 and a molecular weight of 81.757 g/mol. As
a solid, it has a melting point of 648°C and a boiling
point of 1090°C. Its density is 1.738 g/cm3. It reacts in
water to produce dichlorine oxide, the anhydride of
hypochlorous acid.
Mg(ClO2)2+H2O→Mg(OH)2+ Cl2O
Magnesium chlorite can be prepared by reaction of
chlorous acid with magnesium carbonate:
MgCO3(solid)+ 2HClO2 (aq)→Mg(ClO2)2·6H2O
There is a paucity of data regarding this salt and it is
offered for sale only in research quantities. Its solid structure has not been documented.