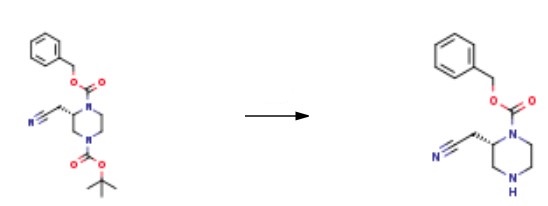
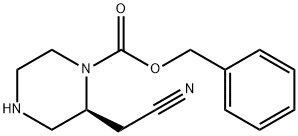
Synthesis
To a solution of I-benzyl 4-tert-butyl (2S)-2-(cyanomethyl) piperazine-1,4-dicarboxylate (6.0 g, 1.0 eq) in dioxane (20.8 mL) was added 4.0 M HCl in dioxane (20.8 mL, 5.0 eq). The mixture was stirred at 20 °C for 1 hour. Then NaHCO3 was added to the reaction mixture until pH>7, after which the reaction was concentrated under reduced pressure to remove dioxane. The residue was diluted with H2O (50 mL) and extracted with ethyl acetate (50 mLx3). The combined organic layers were washed with H2O (20 mL), dried over Na2SO4, filtered, and concentrated under reduced pressure to give a residue. The product benzyl (2S)-2- (cyanomethyl) piperazine-1-carboxylate (4.1 g, 95% yield) was obtained as a yellow oil.