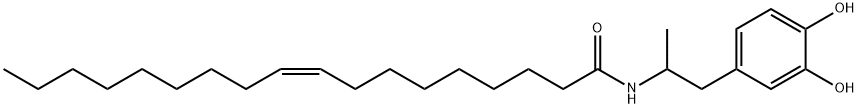
Description
N-(1-(3,4-Dihydroxyphenyl)propan-2-yl)oleamide binds to the cannabinoid 1 (CB
1) receptor with a K
i value of 365 nM in a radioligand binding assay using rat brain homogenate.
1 It has an EC
50 value of 698 nM for the peroxisome proliferator-activated receptor α (PPARα) in a luciferase reporter assay and, in rats, it decreases food intake. It does not inhibit fatty acid amide hydrolase (FAAH).
Uses
OLHHA is a dual CB1 receptor antagonist and PPARα agonist. OLHHA also is a alcohol intake inhibitor with an EC50 value of 0.2 mg/kg. OLHHA reduces both hepatic lipid accumulation and circulating triglyceride levels. OLHHA shows anti-steatotic activity and has the potential for the research of non-alcoholic fatty liver disease (NAFLD)[1][2].
in vivo
OLHHA (5 mg/kg; i.p.; daily for 15 days) reduces both hepatic lipid accumulation and circulating triglyceride levels[2].
| Animal Model: | 8- to 9-week-old male Zucker rats (genetic obesity)[2] |
| Dosage: | 5 mg/kg |
| Administration: | I.p.; daily for 15 days |
| Result: | Reduced the liver fat content and plasma triglyceride levels, and this was accompanied by a general improvement in the profile of plasma parameters related to liver damage in the obese rats, significantly reduced the body weight gain and decreased in the relative expression of CB1 in lean rats. |
References
1. Almeida, B., Joglar, J., Rojas, M.J.L., et al.
Synthesis of fatty acid amides of catechol metabolites that exhibit antiobesity properties. ChemMedChem 4;5(10),1781-1787(2010).