Outline
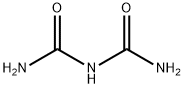
Biuret is the resulting product in urea production process by deamination and condensation reaction when the temperature is higher than the melting point of urea (150-170 ℃). Formula is NH2CONHCONH2. Reaction is as follows NH2CONH2-(heating)-> HNCO + NH3, HNCO + NH2CONH2 → NH2CONHCONH2, crystallization of urea in the granulation process will generate biuret, this substance is toxic to plants, thus content of biuret in urea is required not exceed 1%. Top dressing with urea, the content of biuret should not exceed 0.5%.
Chemical Properties
It is long white sheet crystals. Melting point is 190 ℃ (decomposition), the relative density is 1.467 (-5/4 ℃). Crystals in water contain 4 molecules of water of crystallization, dehydrate at about 110 ℃. It is easily soluble in alcohol, very slightly soluble in ether.
Uses
1. Biuret is used in feed for ruminants, as feed additives as urea, like, but more safe than urea.
2. It is used as pharmaceutical intermediates, growth hormone, foaming agents, paints.
3. It is used for chemical analysis.
Reaction of binary biuret
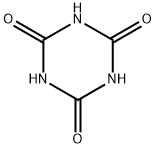
Protein produces purple in alkaline copper sulfate solution, this is because the basic copper sulfate with proteins peptide bond (-NHCO-) formed sake complexes. On the structure of purple product, generally disagree, Xu Fu (Schiff) once separated following the reaction of compound (ⅰ) (purple crystal) from the most simple binary biuret reaction mixture,
the chemical reaction equation related is as follows:
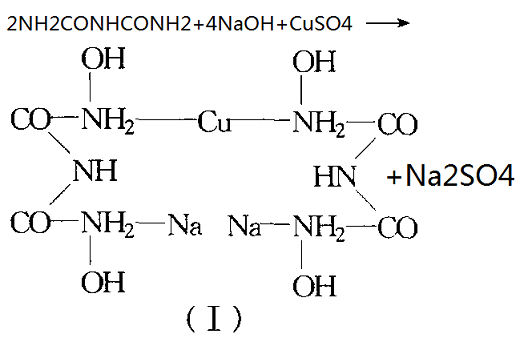
Someone studied with a spectrophotometric method, suggesting that the reaction of binary biuret produces a colored substance, which is mainly composed of a tripeptide and tetrapeptide, complexes produced by ligand of copper and tripeptide was purple, complexes produced by ligand of copper with tetrapeptide was red.
This reaction is not very sensitive, because its peptide bond with another substance that has other groups conduct the biuret reaction. Some substances without the peptide bonds (e.g., histidine) may also conduct this reaction, so its work on the application is limited.
Production method
Mix urea, water with disodium hydrogen phosphate, dissolve by heating. It was heated to 150-160 ℃, heat preservation for 2h. The reaction was completed, the reactants were poured into water and allowed to stand overnight, crystals filtered off were crude, and recrystallize with dilute ammonia to give the finished product of biuret. In the production of urea, intermediate urine is as raw material, by high temperature thermal condensation, separation, and drying, the production is obtained.
Chemical Properties
white powder
Uses
Analytical reagent, especially for proteins.
Uses
Biuret is used in protein assay. It is also used as Catalytic agent, Petrochemical additive. It is used in organic synthesis. Biuret (Carbamoylurea) was used as a source of dietary nitrogen in purified diets for steers.
Definition
A colorless crystalline organic
compound made by heating UREA
(carbamide). It is used in a chemical test for
PROTEINS.
Definition
ChEBI: A member of the class of condensed ureas that is the compound formed by the condensation of two molecules of urea; the parent compound of the biuret group of compounds. Used as a non-protein nitrogen source in ruminant feed.
Agricultural Uses
Biuret is a compound formed from urea when the
temperature during the manufacture of urea goes above
140 to 170°C.
A biuret concentration of more than 2% in urea is
harmful to plants as it affects the metabolism of proteins.
Purification Methods
Crystallise biuret from EtOH. [Beilstein 3 IV 141.]