Manufacturing Process
As described in US Patent 3,123,613, the preparation of the intermediate product, 2-pyridinealdoxime methomethylsulfate, is as follows. 1 kg of 2pyridinealdoxime is dissolved in 6 liters of acetone and filtered until clear. 2 kg (2 equivalents) of freshly distilled dimethyl sulfate are added and the solution mixed. In about 30 minutes crystals start to appear, after which a cooling bath is used to keep the temperature at about 30° to 35°C until the reaction is nearly complete (about 2 hours).
The mixture is allowed to stand at room temperature overnight, the crystals filtered off and washed on a filter with acetone. The product is obtained as colorless needles, which melt at 111° to 112.5°C. The methylsulfate is not stable indefinitely. For preparation of pure chloride salt it is desirable to use methylsulfate which gives no titratable acidity with sodium hydroxide using bromophenol blue as indicator.
10 g of 2-pyridinealdoxime methomethylsulfate are then dissolved in 6 cc of concentrated hydrochloric acid, and 60 cc of isopropanol is added with stirring. Crystals appear almost instantly. After 2 hours standing at room temperature, the crystals are separated by filtration and washed with acetone. The product had a melting point of 227° to 228°C and the yield was 85%.
An alternative route is described in US Patent 3,155,674.
(A) Preparation of 1-Methyl-2-Picolinium Chloride: 98 ml of α-picoline is dissolved in 200 ml of methanol, cooled and 85 ml (at -68°C) of methyl chloride is added. The solution is charged to an autoclave, sealed and the nitrogen pressure of 300 psig is established. The mixture is heated at 120° to 130°C for 2 hours, cooled and opened. The resulting solution is then evaporated to dryness in vacuo, yielding a residue of 110 g. This residue is then dissolved in 50 ml of water and extracted with two 50 ml portions of ether. The aqueous phase is then diluted to 150 ml with water and an assay for ionic chloride is performed which indicates the presence of chloride ion equivalent to 721 mg/ml of l-methyl-2picolinium chloride.
(B) Preparation of 2-(Hydroxyiminomethyl)-1-Methyl Pyridinium Chloride: An aqueous solution of 15 ml of 1-methyl-2-picolinium chloride having a concentration of 477 mg/ml is covered with 50 ml of benzene in an atmosphere of nitrogen and cooled to below 10°C. An aqueous solution of sodium hydroxide is added dropwise and the mixture is stirred for 5 minutes and allowed to stratify. The aqueous phase is then drawn off and the benzenesolution is added slowly to a solution of 3 ml of nitrosyl chloride in 175 ml of benzene containing 0.5 ml of dimethyl formamide at about 10°C in an atmosphere of nitrogen with good agitation. The mixture is then stirred for 1.5 hours and then extracted with four 5 ml of portions of water. The aqueous extracts are then concentrated in vacuo, 30 ml of isopropanol is added and the concentration is repeated. 20 ml of isopropanol is then added to the concentrated mixture, and the mixture is cooled to room temperature and filtered, yielding 3.04 g of crude 2-(hydroxyiminomethyl)-1-methylpyridinium chloride, melting at 202° to 214°C with decomposition. The filtrate is then further concentrated to a 7 g residue which is crystallized from absolute alcohol and yields 0.9 g of 2-(hydroxyiminomethyl)-1-methyl pyridinium chloride melting at 221° to 225°C with decomposition.
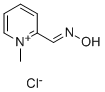
General Description
Pralidoxime chloride, 2-formyl-1-methylpyridinium chloride oxime, 2-PAM chloride,or 2-pyridine aldoxime methyl chloride (Protopamchloride), is a white, nonhygroscopic, crystalline powderthat is soluble in water, 1 g in less than 1 mL.
Clinical Use
Pralidoxime chloride is used as an antidote for poisoningby parathion and related pesticides. It may be effectiveagainst some phosphates that have a quaternary nitrogen. Itis also an effective antagonist for some carbamates, such asneostigmine methylsulfate and pyridostigmine bromide.