Definition
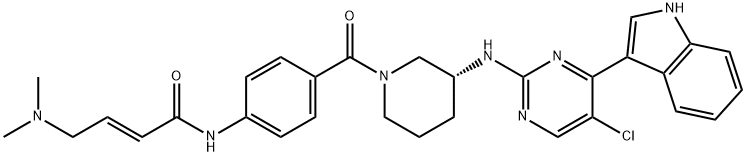
ChEBI: THZ531 is a member of the class of indoles that is 5-chloro-4-(1H-indol-3-yl)-N-[(3R)-piperidin-3-yl]pyrimidin-2-amine in which the piperidine NH group is substituted by a 4-{[(2E)-4-(dimethylamino)but-2-enoyl]amino}benzoyl group. It is a first-in-class CDK12 and CDK13 covalent kinase inhibitor with IC50 of 158 nM and 69 nM, respectively. It has a role as an apoptosis inducer, an antineoplastic agent and an EC 2.7.11.22 (cyclin-dependent kinase) inhibitor. It is a member of indoles, an organochlorine compound, a N-acylpiperidine, an aminopyrimidine, an enamide, a secondary carboxamide and a secondary amino compound.
Biological Activity
thz531 is a first-in-class cdk12 and cdk13 covalent inhibitor with ic50 values of 158 nm and 69 nm, respectively [1].complexes containing cdk12 and cdk13 regulate transcriptional elongation as well as processes, including mrna splicing and 3’-end rna processing. loss of cdk12 and cdk13, or of their cofactor cyclin k, impedes both pol ii processivity and rna processing [1].thz531 is an irreversible cdk12 and cdk13 covalent inhibitor. thz531 potently inhibited cdk12 and cdk13 with ic50 values of 158 nm and 69 nm, whereas inhibition of cdk7 and cdk9 was more than 50-fold weaker, with ic50s of 8.5 μm and 10.5 μm, respectively. in jurkat cells, thz531 irreversibly reduced cell proliferation with ic50 of 50 nm. thz531 induced apoptosis in a dose- and time-dependent way. thz531 also reduced pol ii c-terminal domain (ctd) ser2 phosphorylation, a mark of active transcriptional elongation, resulted in the loss of key super-enhancer-associated transcription factor genes and dna damage response (ddr) genes expression [1].1.zhang t, kwiatkowski n, olson cm, et al. covalent targeting of remote cysteine residues to develop cdk12 and cdk13 inhibitors. nat chem biol. 2016 oct;12(10):876-84.