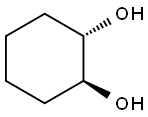
(1S,2S)-TRANS-1,2-CYCLOHEXANEDIOL
- Product Name(1S,2S)-TRANS-1,2-CYCLOHEXANEDIOL
- CAS57794-08-8
- CBNumberCB8265320
- MFC6H12O2
- MW116.16
- MDL NumberMFCD00066431
- MOL File57794-08-8.mol
- MSDS FileSDS
Chemical Properties
| Melting point | 107-109 °C(lit.) |
| Boiling point | 177.22°C (rough estimate) |
| Density | 0.9958 (rough estimate) |
| refractive index | 1.4270 (estimate) |
| Flash point | 134 °C |
| pka | 14.49±0.40(Predicted) |
| optical activity | [α]20/D +39°, c = 1.6 in H2O |
| BRN | 1901343 |
| FDA UNII | 3RX9RD438J |
Safety
| Symbol(GHS) |
 
|
| Signal word | Danger |
| Hazard statements | H318-H335 |
| Precautionary statements | P261-P280-P305+P351+P338 |
| Hazard Codes | Xi |
| Risk Statements | 36/37-41 |
| Safety Statements | 26-39 |
| WGK Germany | 3 |
| HS Code | 29061990 |