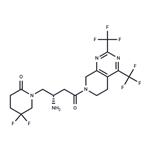
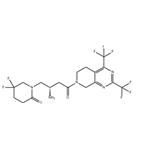
Description
Gemigliptin is a prolyl-specific dipeptidyl aminopeptidase IV
(DPP IV, DPP-4, CD26) inhibitor approved for the treatment of type
2 diabetes mellitus by the Korean Food and Drug Administration in
2012. Gemigliptin was discovered and developed by LG Life
Sciences and is now the sixth DPP-4 inhibitor approved for the
treatment of type 2 diabetes. At the time this review was prepared,
there were no publications describing the discovery strategy
and preclinical data that led to the advancement of gemigliptin to the clinic.
Synthesis
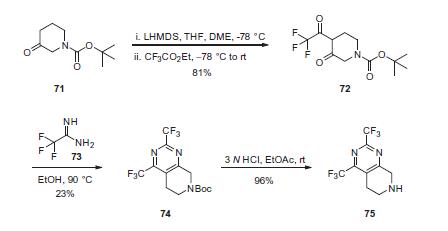
Commercial N-Boc-3-piperidone (71) was treated
with LHMDS followed by ethyl trifluoroacetate to effect a Claisen
condensation, producing diketone 72 in 81% yield. Cyclization of
72 with 2,2,2-trifluoroacetamide (73) gave bis-trifluoromethyl
dihydropyridopyrimidine 74 in 23% yield. Removal of the Boc protecting
group efficiently provided amine 75 in 96% yield.
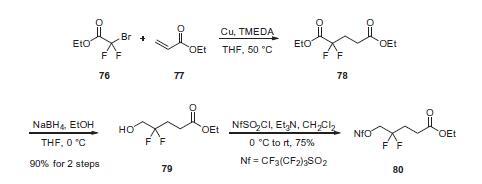
1,4-Addition of ethyl bromodifluoroacetate
(76) to ethyl acrylate (77) in the presence of
copper powder and tetramethylethylenediamine (TMEDA) gave
diester 78, which was selectively reduced with sodium borohydride
(NaBH4) to give alcohol 79 in 90% overall yield for the
two-step procedure. Alcohol 79 was then treated with perfluorobutanesulfonyl
chloride and triethylamine to give activated
alcohol 80 in 75% yield.
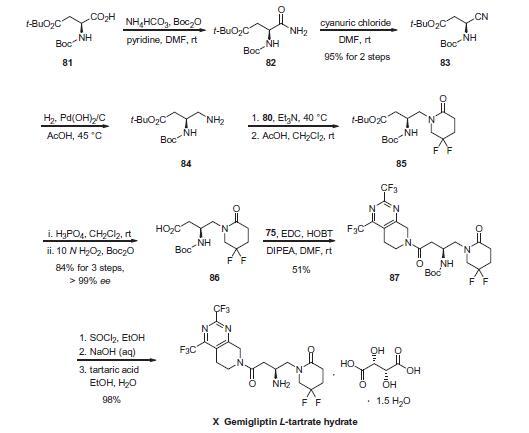
Boc-L-aspartic acid 4-tert-butyl ester (81) was treated
with ammonium bicarbonate and pyridine in the presence of
di-tert-butyl dicarbonate to give formamide 82. Dehydration of
82 to give nitrile 83 was accomplished through reaction with cyanuric
chloride in 95% overall yield for the two-step sequence.
Hydrogenation of 83 in the presence of Pearlman?ˉs catalyst provided butyl amine 84. Alkylation of 84 with activated alcohol
80 in triethylamine followed by cyclization in acetic acid afforded
difluoropyridone 85. Acidic hydrolysis of the ester proceeded with
concomitant removal of the Boc protecting group, and was followed
by reprotection of the amine with di-tert-butyl dicarbonate
to give acid 86 in 84% overall yield for the three-step procedure in
>97% ee. Coupling of 86 with fragment 75 in the presence of 1-hydroxybenzotriazole
(HOBT) and 1-ethyl-3-(3-dimethylaminopropyl)
carbodiimide (EDC) gave amide 87 in 51% yield. Removal
of the Boc group with thionyl chloride in ethanol followed by neutralization
with aqueous sodium hydroxide and salt formation
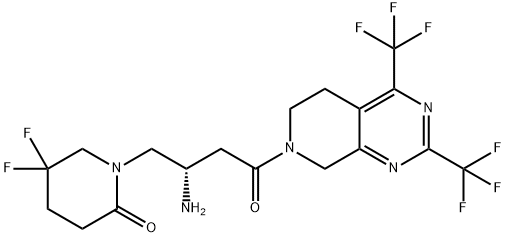
with L-tartaric acid provided gemigliptin L-tartrate hydrate (X) in
97.5% yield.
 1,4-Addition of ethyl bromodifluoroacetate
(76) to ethyl acrylate (77) in the presence of
copper powder and tetramethylethylenediamine (TMEDA) gave
diester 78, which was selectively reduced with sodium borohydride
(NaBH4) to give alcohol 79 in 90% overall yield for the
two-step procedure. Alcohol 79 was then treated with perfluorobutanesulfonyl
chloride and triethylamine to give activated
alcohol 80 in 75% yield.
1,4-Addition of ethyl bromodifluoroacetate
(76) to ethyl acrylate (77) in the presence of
copper powder and tetramethylethylenediamine (TMEDA) gave
diester 78, which was selectively reduced with sodium borohydride
(NaBH4) to give alcohol 79 in 90% overall yield for the
two-step procedure. Alcohol 79 was then treated with perfluorobutanesulfonyl
chloride and triethylamine to give activated
alcohol 80 in 75% yield.
 Boc-L-aspartic acid 4-tert-butyl ester (81) was treated
with ammonium bicarbonate and pyridine in the presence of
di-tert-butyl dicarbonate to give formamide 82. Dehydration of
82 to give nitrile 83 was accomplished through reaction with cyanuric
chloride in 95% overall yield for the two-step sequence.
Hydrogenation of 83 in the presence of Pearlman?ˉs catalyst provided butyl amine 84. Alkylation of 84 with activated alcohol
80 in triethylamine followed by cyclization in acetic acid afforded
difluoropyridone 85. Acidic hydrolysis of the ester proceeded with
concomitant removal of the Boc protecting group, and was followed
by reprotection of the amine with di-tert-butyl dicarbonate
to give acid 86 in 84% overall yield for the three-step procedure in
>97% ee. Coupling of 86 with fragment 75 in the presence of 1-hydroxybenzotriazole
(HOBT) and 1-ethyl-3-(3-dimethylaminopropyl)
carbodiimide (EDC) gave amide 87 in 51% yield. Removal
of the Boc group with thionyl chloride in ethanol followed by neutralization
with aqueous sodium hydroxide and salt formation
with L-tartaric acid provided gemigliptin L-tartrate hydrate (X) in
97.5% yield.
Boc-L-aspartic acid 4-tert-butyl ester (81) was treated
with ammonium bicarbonate and pyridine in the presence of
di-tert-butyl dicarbonate to give formamide 82. Dehydration of
82 to give nitrile 83 was accomplished through reaction with cyanuric
chloride in 95% overall yield for the two-step sequence.
Hydrogenation of 83 in the presence of Pearlman?ˉs catalyst provided butyl amine 84. Alkylation of 84 with activated alcohol
80 in triethylamine followed by cyclization in acetic acid afforded
difluoropyridone 85. Acidic hydrolysis of the ester proceeded with
concomitant removal of the Boc protecting group, and was followed
by reprotection of the amine with di-tert-butyl dicarbonate
to give acid 86 in 84% overall yield for the three-step procedure in
>97% ee. Coupling of 86 with fragment 75 in the presence of 1-hydroxybenzotriazole
(HOBT) and 1-ethyl-3-(3-dimethylaminopropyl)
carbodiimide (EDC) gave amide 87 in 51% yield. Removal
of the Boc group with thionyl chloride in ethanol followed by neutralization
with aqueous sodium hydroxide and salt formation
with L-tartaric acid provided gemigliptin L-tartrate hydrate (X) in
97.5% yield.