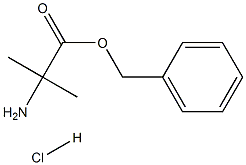
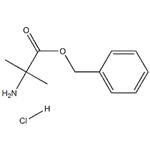
Chemical Properties
Benzyl 2-amino-2-methylpropanoate hydrochloride, also known as H-Aib-OBzl, is a white solid.
Preparation
A mixture of 2-amino-2- methylpropanoic acid (3 g, 29.1 mmol), benzyl alcohol (12 mL, 115 mmol), and p- toluenesulfonic acid monohydrate (5.53 g, 29.1 mmol) in toluene (40 mL) was heated under reflux overnight. Additional benzyl alcohol (12 mL, 115 mmol) was added and the reaction mixture was heated with a Dean Starck overnight. The reaction mixture was concentrated under reduced pressure and washed with diethyl ether. Diethyl ether was removed, and then the residue was dissolved in ethyl acetate and washed with a saturated aqueous NaHC03 solution. The organic layer was dried over Na2S04 and concentrated under reduced pressure. The resulting product was triturated with diethyl ether and HCI in dioxane. The solid was filtered, washed with diethyl ether and dried under reduced pressure to afford benzyl 2-amino-2-methylpropanoate hydrochloride (H-Aib-OBzl).
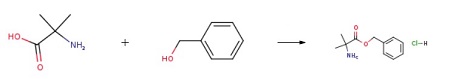
reaction suitability
reaction type: solution phase peptide synthesis
Waste Disposal
The material can be disposed of by removal to a licensed chemical destruction plant or by controlled incineration with flue gas scrubbing. Do not contaminate water, foodstuffs, feed or seed by storage or disposal. Do not discharge to sewer systems.