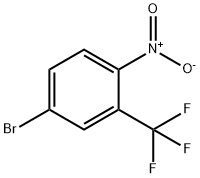
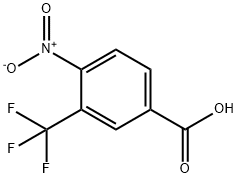
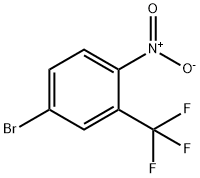
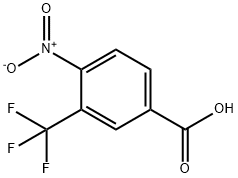
Synthesis
GENERAL PROCEDURE: To a 25 mL round-bottomed flask fitted with a Dimroth condenser (cooled to 10°C) was added 4-nitro-3-(trifluoromethyl)benzoic acid (1.8 mmol), chloroisocyanurate, brominating agent and solvent (8 mL). The mixture was stirred and heated in an oil bath under fluorescent room light (FL) illumination. Upon completion of the reaction, the cooled reaction mixture was filtered through a short silica gel pad, washed with 1 M Na2SO3 aqueous solution, dried over anhydrous Na2SO4, filtered and concentrated in vacuum to give 5-bromo-2-nitrobenzotrifluoride. The resulting product contained 1-5% of the corresponding chlorinated nitro compounds as by-products. The experimental results are detailed in Table 2. Table 2. Results of bromocarboxylation reaction of nitroaromatic carboxylic acids.