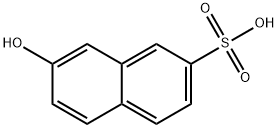
Chemical Properties
2-Hydroxynaphthalene-7-sulfonic acid [92- 40-0]. (7-hydroxynaphthalene-2-sulfonic acid), F acid, Cassella acid, C10H8O4S, Mr 224.23, is soluble in water, from which it can be recrystallized as the monohydrate (mp 89℃). The sodium and potassium salts are also readily soluble, but the barium salt is sparingly soluble. Iron(III) chloride gives a dark blue color with neutral solutions of 2-Hydroxynaphthalene-7-sulfonic acid. Azo coupling and nitrosation occurs in the 1-position. Sulfonation gives a variety of products depending on the conditions.
Uses
2-Hydroxynaphthalene-7-sulfonic acid is used as an intermediate in the production of 2-aminonaphthalene-7-sulfonic acid, 2-hydroxynaphthalene-3,7-disulfonic acid, and 2,7-dihydroxynaphthalene. It has minor uses as an azo coupling component.
Preparation
Naphthalene-2,7-disulfonic acid is heated with aqueous sodium hydroxide under pressure at 230℃ for 5 h and then at 260℃ for another 5 h. After neutralizing and screening, the liquor is acidified to precipitate the product, which is isolated after boiling off the sulfur dioxide and cooling to 30℃.