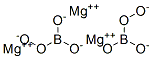Chemical Properties
White powder. Sparingly soluble in
water; decomposes with evolution of oxygen.
Physical properties
Magnesium perborate is a bulky, white powder which
gradually decomposes in air with loss of active oxygen.
It is slightly soluble in water, with partial decomposition,
so that it has been used in medicine as an oxidizing
agent, antiseptic and antibacterial agent. Magnesium perborate is soluble in
dilute acids.
Uses
Their main
usage has been in dental preparations and in laundry
detergents as cleaning and bleaching agents.
Uses
Driers, bleaching, antiseptic (tooth powders).
Preparation
Magnesium perborate, Mg(BO
3)
2, can also be
prepared by a solution method:
MgCl
2 (aq) + Na
2BO
3 (aq)→Mg(BO
3)
2·7H
2O
Hazard
Moderate fire risk in contact with organic
materials.