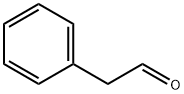
Occurrence
Identified among the constituents of several essential oils: neroli, Citrus sinensis leaves, other citrus species
(flowers and leaves), narcissus, magnolia, lily, rose and tea. It is reported found in over 170 natural products including apricot,
sour cherry, cooked apple, peach, fresh blackberry, crispbread, other types of bread, green tea, unprocessed rice, lemon balm, red
sage, black currant, bilberry, cranberry, other berries, grapes, raisins, melon, papaya, guava fruit, pineapple, asparagus, celery
leaves, carrot, parsley, peas, bell pepper, sweet pepper, peach, cabbage, peppermint oil, Scotch spearmint oil, mustard, vinegar,
onion, cooked potato, tomato, cinnamon bark, cassia leaf, ginger, many cheeses, milk, yogurt, boiled egg, cooked and cured
meats, beer, cognac, grape wines, cocoa, coffee, tea, roasted filbert, roasted peanut, soybean, pecans, cauliflower, broccoli, honey,
avocado, passion fruit, beans, mushrooms, trassi, mango, tamarind, rice, licorice, buckwheat, lovage root, pumpkin, sweet potato,
cassava, corn oil, malt, wort, dried bonito, loquat, pawpaw, maté, sweet grass oil, orange peel oil, grapefruit juice, endive, clam
and Chinese quince.