Synthesis
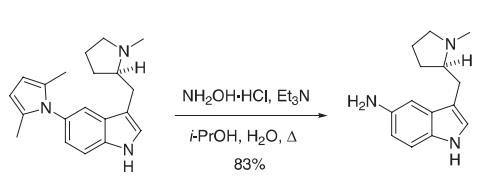
A mixture of the 2,-5-dimethylpyrrole (81.5 g, 0.265 mol), hydroxylamine hydrochloride (368 g, 5.30 mol), and triethylamine (367 mL, 2.65 mol) in 2-propanol (800 mL) and water (200 mL) was heated at reflflux under nitrogen for 4.5 h. The resulting reaction mixture was cooled in an ice bath, solid sodium hydroxide (212 g, 5.30 mol) was added, and the resulting reaction mixture was stirred at room temperature under nitrogen for 24 h. The reaction mixture was then fifiltered through Celite, and the fifiltrate was evaporated under reduced pressure. The residual oil was passed through a silica gel fifilter (~ 1 kg) followed by elution with EtOAc/MeOH/Et3N (8:1:1) to afford 85 g of a pale yellow solid. The solid was dissolved in EtOAc (1 L), and this solution was washed with a saturated solution of sodium chloride (3 × 100 mL). The organic layer was dried (Na2SO4) and evaporated under reduced pressure to afford 50.55 g (83%) of the 5-aminoindole. Reference: Macor, J. E.; Chenard, B. L.; Post, R. J. J. Org. Chem. 1994, 59, 7496−7498.

