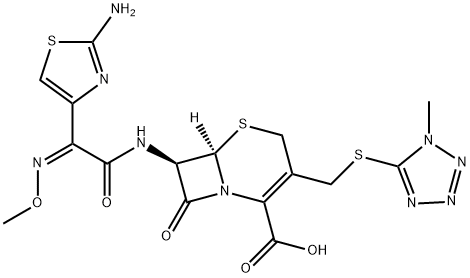
Cefmenoxime
- Product NameCefmenoxime
- CAS65085-01-0
- CBNumberCB7771510
- MFC16H17N9O5S3
- MW511.56
- EINECS278-299-4
- MDL NumberMFCD00864851
- MOL File65085-01-0.mol
- MSDS FileSDS
Chemical Properties
| Density | 1.96±0.1 g/cm3(Predicted) |
| pka | 2.61±0.50(Predicted) |
| CAS DataBase Reference | 65085-01-0(CAS DataBase Reference) |
| FDA UNII | KBZ4844CXN |
| ATC code | J01DD05 |
Cefmenoxime Chemical Properties,Usage,Production
Originator
Tacef,Takeda,W. Germany,1983Uses
Antibacterial.Uses
Cefmenoxime (cas# 65085-01-0) is a compound useful in organic synthesis.Definition
ChEBI: A third-generation cephalosporin antibiotic, bearing a 2-(2-amino-1,3-thiazol-4-yl)-2-(methoxyimino)acetyl]amino group at the 7beta-position and a [(1-methyl-1H-tetrazol-5-yl)sulfanyl]methyl group at the 3-position.Manufacturing Process
7β-[α-Methoxyimino-α-(2-aminothiazol-4-yl)acetamido]cephalosporanicacid trifluoroacetic acid salt is dissolved in a solution of 272 mg of 1-methyl-5- mercapto-1H-tetrazole, 555 mg of sodium bicarbonate and 68 mg of triethylbenzylammonium bromide in 10 ml of water. The solution is heated at 60°C in nitrogen atmosphere for 6 hours. After cooling, the reaction solution is passed through a column of Amberlite XAD-2 and eluted with water and then with 2.5% ethanol. The procedure yields sodium 7β-[α-methoxyimino-α- (2-aminothiazol-4-yl)acetamido]-3-(1-methyl-1H-tetrazol-5-ylthiomethyl)-3- cephem-4-carboxylate, MP 174°C to 175°C (decomposition).brand name
Cefmax (TAP).Therapeutic Function
AntibacterialAntimicrobial activity
A semisynthetic cephalosporin supplied as the hydrochloride. Its activity is very similar to that of cefotaxime. A 500 mg intramuscular injection achieves a plasma concentration of 15 mg/L after 40 min. A concentration of 200 mg/L is attained after intravenous administration of 1 g. The plasma half-life is c. 1 h. Around 77% is protein bound. Probenecid increases peak plasma levels and extends the plasma half-life to 1.8 h. Therapeutic concentrations are achieved in CSF. There is a degradation product with a long half-life (around 40 h), but 80–92% of the drug is recovered unchanged from the urine. In patients with renal insufficiency, no significant relation was found between creatinine clearance and peak serum concentrations but there was a linear relationship with plasma half-life and total body clearance. About 10% of the dose appears in the feces, mostly extensively degraded, possibly by the fecal flora.Toxicity, side effects and clinical use are those common to group 4 cephalosporins.
Preparation Products And Raw materials
Cefmenoxime Supplier
Global(92)Suppliers
| Supplier | Tel | Country | ProdList | Advantage | |
|---|---|---|---|---|---|
| +86-15531157085 +86-15531157085 |
abby@chuanghaibio.com | China | 8808 | 58 | |
| +86 13288715578 +8613288715578 |
sales@hbmojin.com | China | 12815 | 58 | |
| +86-21-33585366 - 03@ | sales03@shyrchem.com | CHINA | 738 | 60 | |
| +8618523575427 | sales@conier.com | China | 49732 | 58 | |
| +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29808 | 58 | |
| 13180553332 | 2179877681@qq.com | CHINA | 990 | 58 | |
| +1-781-999-5354 +1-00000000000 |
marketing@targetmol.com | United States | 32159 | 58 | |
| +8618032935937 18032916000 |
demi@hbtycoon.com | CHINA | 448 | 58 | |
| +86-029-89586680 +86-18192503167 |
1026@dideu.com | China | 7813 | 58 | |
| +86-0571-85134551 | sales@afinechem.com | China | 15350 | 58 |
65085-01-0, CefmenoximeRelated Search
- 7-(5-amino-5-carboxyvaleramido)cephalosporanic acid
- 2-Methyl-4-isothiazolin-3-one
- Cephalexin
- PENICILLINASE
- Ceftizoxime sodium
1of4
PROMPT×
PROMPT
The What'sApp is temporarily not supported in mainland China
The What'sApp is temporarily not supported in mainland China
Cancel
Determine

