Uses
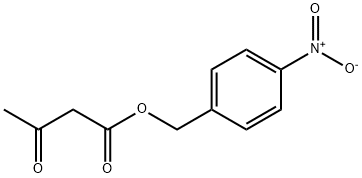
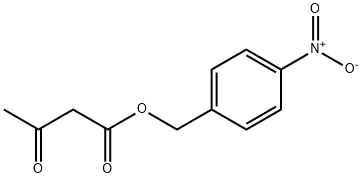
4-Nitrobenzyl Acetoacetate acts as a reagent in the preparation of panipenem via esterification of nitrobenzyl alcohol followed by substitution, cyclizations and hydrogenation. Panipenem I is a new carbapenem antibiotic for the treatment of inflammation.
Synthesis
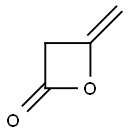
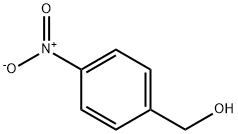
GENERAL STEPS: 4-Nitrobenzyl alcohol (1.53 g, 10 mmol) was placed in a round bottom flask and dichloromethane (10 mL) was added. Bis(vinylidene) ketone (1.26 g, 15 mmol) was slowly added followed by triethylamine (0.14 mL, 1 mmol) under cooling in an ice bath. The reaction mixture was warmed up to room temperature with continuous stirring for 3 hours. Upon completion of the reaction, it was quenched by the addition of water (10 mL). The organic layer was separated, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure to afford 4-nitrobenzyl acetoacetate (2.30 g, 97% yield). Referring to the method of Example 1, the resulting 4-nitrobenzyl acetoacetate was further reacted to produce ethyl p-nitrobenzyl acetoacetate (2.09 g, 68% yield). The structure of the product was confirmed by 1H NMR (CDCl3) and mass spectrum: 1H NMR (CDCl3) δ 8.25 (d, 2H), 7.53 (s, 1H), 7.51 (d, 2H), 6.57 (s, 1H), 5.27 (s, 2H), 4.21 (s, 2H), 2.54 (s, 3H); MS (M+1): 308.