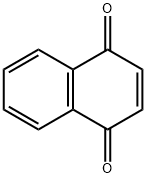
Chemical Properties
1,4-Naphthoquinone is a yellow to greenish yellow crystalline solid that has pungent odor like benzoquinone. It is slightly soluble in water, soluble in ether, chloroform, benzene, glacial acetic acid. In alkaline solutions it produces a reddish-brown color.
Uses
antibacterial, antineoplastic
Uses
1,4-Naphthoquinone is used as a strong dienophile in Diels-Alder reaction. It is also used as a precursor to anthroquinone and 5-nitro-1,4-naphthalenedione, which finds application in the preparation of aminoanthroquinone and is also used as a dye precursor. It is a basic component of Vitamin K.
Definition
ChEBI: 1,4-naphthoquinone is the parent structure of the family of 1,4-naphthoquinones, in which the oxo groups of the quinone moiety are at positions 1 and 4 of the naphthalene ring. Derivatives have pharmacological properties. It derives from a hydride of a naphthalene.
Application
1,4-Naphthoquinone is an important organic intermediate compound, it is the chemical intermediate of fungicide dichlone and dithianon, and also the by-product of anthraquinone synthesis. It can be used as polymerisation regulator of rubber and polyester resin, synthesis of dyestuffs and drugs, algaecide, corrosion inhibitor, stabiliser of transformer oil, etc.
Origin
1,4-Naphthoquinone is a natural product that is widely distributed in nature and is found in black walnut and king walnut. Especially in several families of higher plants (like Plumbaginaceae, Juglandaceae, Ebenaceae, Boraginaceae, Dioncophyllaceae, Ancistrocladaceae, Iridaceae, Verbenaceae, Scrophulariaceae, Avicenniaceae, Balsaminaceae, Bignoniaceae, Gentianaceae, and Droseraceae), and in addition also being present in algae, fungi, some animals, and products of metabolism in some bacteria.
General Description
Yellow needles or brownish green powder with an odor of benzoquinone.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
1,4-Naphthoquinone may react with many acids and bases liberating heat and flammable gases (e.g., H2). The heat may be sufficient to start a fire in the unreacted portion of the ketone. May react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. May react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4.
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition 1,4-Naphthoquinone emits toxic fumes and smoke.
Fire Hazard
Flash point data for 1,4-Naphthoquinone are not available; however, 1,4-Naphthoquinone is probably combustible.
Flammability and Explosibility
Not classified
Safety Profile
Poison by ingestion,
intravenous, subcutaneous, and
intraperitoneal routes. Experimental
reproductive effects. Questionable
carcinogen with experimental tumorigenic
data. When heated to decomposition it
emits acrid smoke and irritating fumes.
Synthesis
1,4-Naphthoquinone is prepared by reacting naphthalene with molecular oxygen in a gaseous phase in the presence of a catalyst containing vanadium.
Prior to reacting the naphthalene with oxygen, the catalyst is pre-treated with molecular oxygen at 300° to 400° C in the absence of organic compounds and immediately thereafter a gas mixture containing naphthalene and molecular oxygen is passed over the pretreated catalyst at temperatures in the range of 300° to 400° C. The catalyst pre-treatment can be carried out in the presence of water vapor and the subsequent reaction of naphthalene with molecular oxygen can also be carried out in the presence of water vapor.
Potential Exposure
1,4-Naphthoquinone is used as a polymerization
regulator for rubber and polyester resins; in the
synthesis of dyes and pharmaceuticals; and as a fungicide
and algicide.
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard
Class: 6.1; Labels: 6.1-Poisonous materials, Technical
Name Required.
Purification Methods
Crystallise the quinone from diethyl ether (charcoal). It distils in steam. It also crystallises from *benzene or aqueous EtOH and sublimes in a vacuum. [Beilstein 7 IV 2422.]
Incompatibilities
Ketones are incompatible with oxidizers
(chlorates, nitrates, peroxides, permanganates, perchlorates,
chlorine, bromine, fluorine, etc.); contact may cause fires
or explosions. Keep away from alkaline materials, strong
bases, strong acids, oxoacids, epoxides, nitrated amines,
azo, diazo, azido compounds, carbamates, organic cyanates.
May react with many acids and bases liberating heat and
flammable gases (e.g., hydrogen) generating heat may be
sufficient to start a fire in the unreacted portion of the
ketone. May react with reducing agents such as hydrides,
alkali metals, and nitrides to produce flammable gas
(e.g., hydrogen) and heat. Incompatible with isocyanates,
aldehydes, cyanides, peroxides, and anhydrides. May react
violently with aldehydes.
Waste Disposal
Consult with environmental
regulatory agencies for guidance on acceptable disposal
practices. Generators of waste containing this contaminant
(≥100 kg/mo) must conform with EPA regulations governing
storage, transportation, treatment, and waste disposal.