Synthesis
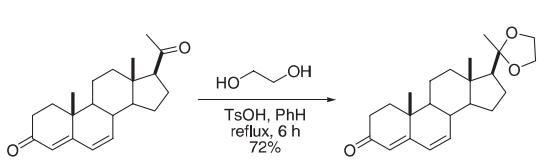
A mixture of pregna-4,6-diene-3,20-dione (2.70 g, 8.64 mmol), ethylene glycol (5.0 mL), p-TsOH (100 mg, 0.52 mmol) and benzene (150 mL) was stirred and heated under reflflux for 6 h. Water formed during the reaction was removed by a Dean–Stark trap. The cooled reaction mixture was diluted with Et2O (600 mL) and sequentially washed with saturated aqueous NaHCO3, water, and brine. The Et2O extract was stirred and dried over anhydrous MgSO4 (35 g, 0.29 mol) for several hours until thin layer chromatography showed that the ketal group at C-3 was hydrolyzed completely. The mixture was fifiltered and the solvent was evaporated. The residue was purifified by silica gel column chromatography to give the 1,3-dioxolane product (2.32 g, 75%) as a white solid. Reference: Zeng, C.-M.; Manion, B. D.; Benz, A.; Evers, A. S.; Zorumski, C. F.; Mennerick, S.; Covey, D. F. J. Med. Chem. 2005; 48; 3051−3059.

