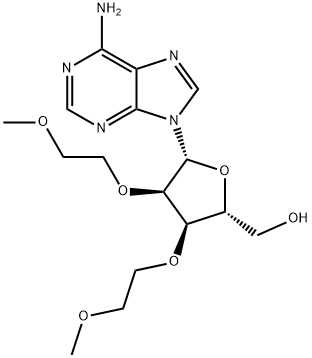
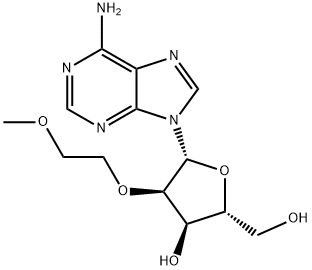
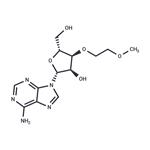
Synthesis
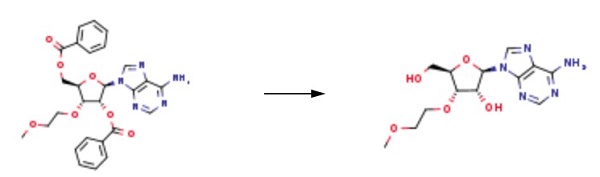
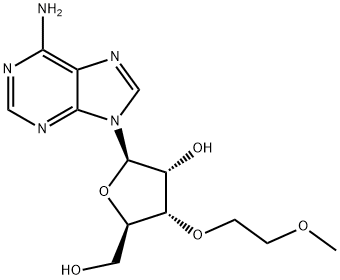
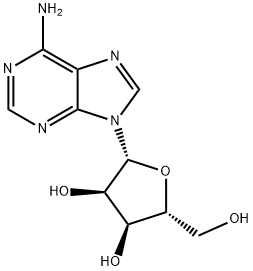
26.0 g of ((2R,3R,4R,5R)-5-(6-amino-9H-purin-9-yl)-4-(benzoyloxy)-3-(2-methoxyethoxy)tetrahydrofuran-2-yl)methyl benzoate was weighed, and 8.1 g of adenine was suspended in 250 mL of acetonitrile and 1,2-dichloroethane, and the temperature was lowered to 10 ° C. Add 26.1g of tin tetrachloride. After the completion of the dropwise addition, the reaction was stirred at 25 °C. After the completion of the reaction by HPLC, it was quenched with water. The reaction mixture was diluted with methylene chloride, washed with water, and evaporated. The evaporated crude product was dissolved in 200 mL of methanol / aqueous ammonia and stirred at 25 ° C overnight. After completion of the reaction, the mixture was concentrated and purified to give the product 3'-O-methoxyethyl adenosine 12.1 g, purity 99.6%, yield 70%.