Description
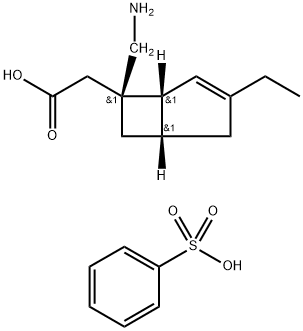
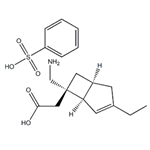
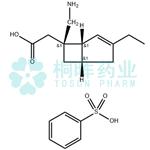
Mirogabalin besylate (mirogabalin, Tarlige) is a gabapentinoid therapy developed by Daiichi Sankyo, which is approved in Japan for the treatment of postherpetic neuralgia and painful diabetic peripheral neuropathy. Mirogabalin has a potent pain-modulating effect with a unique high affinity and prolonged dissociation rate for the a2delta-1 subunit of voltage-gated calcium (Ca2+) channels (VGCCs) on the dorsal root ganglion resulting in more sustained analgesia compared with traditional gabapentinoids[1].
Uses
Mirogabalin besylate is an oral gabapentin analogue for the treatment of peripheral neuralgia (PNP), including diabetic PNP and postherpetic neuralgia. The drug has been approved in Japan for the treatment of PNP and has not yet received FDA approval.
Mechanism of action
Mirogabalin besylate selectively and potently binds to the α2δ subunit of voltage-gated calcium channels. When mirogabalin is bound, the influx of calcium ions to the presynaptic terminal is reduced, thereby inhibiting the release of excitatory neurotransmitters and providing analgesic effects.
Side effects
Mirogabalin has a superior adverse events (AEs) profile due to a rapid dissociation from the a2delta-2 subunit of VGCCs potentially implicated in central nervous system-specific AEs. The most common AEs for mirogabalin are dizziness (approximately 8-16%), somnolence (approximately 6-24%) and headache (approximately 6-14%), with a lower incidence of constipation, nausea, diarrhea, vomiting, edema, fatigue and weight gain[1].
Metabolism
Mirogabalin is rapidly absorbed, and clearance is through renal excretion (approximately 80%) with approximately 20% being metabolized. Metabolism is primarily to the biologically inactive14 lactam form (A204-4455) and involves various uridine 5′-diphospho-glucuronosyltransferase (UGT) isoforms acting at the carboxylic acid moiety to produce mirogabalin acylglucuronide, which is then nonenzymatically converted to A204-4455.
References
[1] J Burgess. “Mirogabalin besylate in the treatment of neuropathic pain.” Drugs of today 56 2 (2020): 135–149.