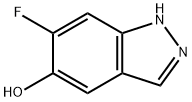
Synthesis
In a pressure vessel intermediate 3 (0.659g, 4.67 mmol) and
potassium acetate (11.39 mmol, 1.12 g) were dissolved in chloroform (30 mL) at 0⁰C. Then
acetic anhydride (21.01 mmol, 1.98 mL) was added drop wise to the reaction. The reaction was
then warmed to room temperature and stirred for 30 min. The reaction was then heated to 80 ⁰C
and isopentyl nitrite (5.14 mmol, 0.69 mL) was added drop wise.The reaction was further stirred
at 80 ⁰C overnight turning a dark brown color. The reaction was neutralized with NaHCO3 to pH
7. Dichloromethane was then added to dilute the reaction and the layers were separated. The
organic layer was then washed 2x with NaCl, dried over MgSO4, and concentrated. Dissolved
resulting residue in MeOH (15 mL) and 6N HCl (15 mL) and refluxed overnight at 40 °C.
Neutralized the reaction with NaOH and then concentrated off the MeOH. Extracted the aqueous
layer with ethyl acetate 2x, then washed the ethyl acetate 2x with NaCl, dried over MgSO4, and
concentrated to give a brown residue. Purified the resulting residue using a gradient of 40% -
100% EtOAc/Hexanes to give 6-fluoro-1H-indazol-5-ol (4b) (1.571 g, 60%).1H NMR (400 MHz, DMSO-d6) δ 12.83 (s, 1H), 9.55 (s, 1H), 7.88 (s, 1H), 7.30 (dd, J = 10.9, 1.1 Hz, 1H), 7.17
(d, J = 8.5 Hz, 1H).