Uses
TY 52156 is a sphingosine-1-phosphate receptor subtype 3 (S1PR3) antagonist, which is used for the treatment of lung cancer.
Definition
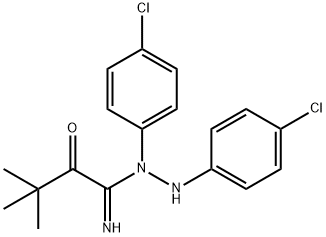
ChEBI: TY 52156 is an imidohydrazide resulting from the formal condensation of the hydrazide carbonyl group of N'-(p-chlorophenyl)-3,3-dimethyl-2-oxobutanehydrazide with the amino group of p-chloroaniline. A potent and selective antagonist of sphingosine-1-phosphate receptor 3 (S1PR3). It has a role as a sphingosine-1-phosphate receptor 3 antagonist. It is a member of monochlorobenzenes and an imidohydrazide.
Biological Activity
ty 52156 (1-(4-chlorophenylhydrazono)-1-(4-chlorophenylamino)-3,3-dimethyl-2-butanone) is a novel s1p3 receptor antagonist [1].sphingosine 1-phosphate (s1p) is a bioactive lysophospholipid mediator mainly released from activated platelets and implicated in many biological responses, such as angiogenesis, vascular development, and cardiovascular function [1].
in vitro
ty-52156 preferentially inhibited the s1p-induced increase in [ca2+]i in s1p3-cho cells. ty-52156 competitively inhibited the dose-dependent [ca2+]i increase elicited by s1p in s1p3-cho with the ki value of ~110 nm for s1p3 receptor. ty-52156 showed submicromolar potency and a high degree of selectivity for s1p3 receptor. ty-52156 (10 μm) inhihbited 24 gpcrs and three ion channels by 〈 30%. s1p dose-dependently decreased cf (coronary flow) in perfused rat heart. s1p dose-dependently induced vasoconstriction in isolated canine cerebral arteries. in hcasmcs, ty-52156 inhibited s1p-induced rho activation.
in vivo
in sd rats, oral bioavailability of ty-52156 was ~70.9%. in rats, pretreatment with ty-52156 significantly attenuated fty-720-induced bradycardia, a broad agonist of s1p receptors. pretreatment with ty-52156 prevented the fty-720-p-induced increase in [ca2+]i in a dose-dependent manner.
References
[1] murakami a, takasugi h, ohnuma s, et al. sphingosine 1-phosphate (s1p) regulates vascular contraction via s1p3 receptor: investigation based on a new s1p3 receptor antagonist[j]. molecular pharmacology, 2010, 77(4): 704-713.