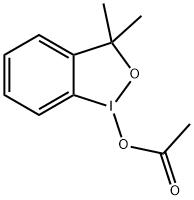
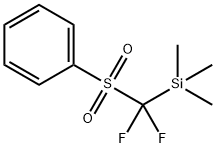
Description
3,3-Dimethyl-1-[difluoro(phenylsul
fonyl)methyl]-1,2-benziodoxole is a novel electrophilic difluoromethylation reagent prepared from TMSCF2SO2Ph, which can
efficiently transfer the PhSO2CF2 moiety to nucleophiles such as thiols under mild reaction
conditions. Copper(II)-catalyzed decarboxylative difluoro(phenylsulfonyl)methylation of α,β- or
β,γ-unsaturated carboxylic acids with this reagent can afford vinylic and allylic
difluoromethylation products.
Reactions
(1) Difluoromethylation of thiols.
![3,3-DiMethyl-1-[difluoro(phenylsulfonyl)Methyl]-1,2-benziodoxole 3,3-DiMethyl-1-[difluoro(phenylsulfonyl)Methyl]-1,2-benziodoxole](/NewsImg/2024-01-23/6384160274293716773902182.png)
(2) Difluoromethylation of β,γ-unsaturated carboxylic acids.
![3,3-DiMethyl-1-[difluoro(phenylsulfonyl)Methyl]-1,2-benziodoxole 3,3-DiMethyl-1-[difluoro(phenylsulfonyl)Methyl]-1,2-benziodoxole](/NewsImg/2024-01-23/6384160275195768365581876.png)
(3) Difluoromethylation of α,β-unsaturated carboxylic acids.
![3,3-DiMethyl-1-[difluoro(phenylsulfonyl)Methyl]-1,2-benziodoxole 3,3-DiMethyl-1-[difluoro(phenylsulfonyl)Methyl]-1,2-benziodoxole](/NewsImg/2024-01-23/6384160276009714922267705.png)
References
[1] WEI ZHANG; Jinbo H; Jieming Zhu. Electrophilic (phenylsulfonyl)difluoromethylation of thiols with a hypervalent iodine(III)–CF2SO2Ph reagent[J]. Tetrahedron Letters, 2008. DOI:
10.1016/j.tetlet.2008.06.064.[2] ZHENGBIAO HE. Copper-Catalyzed Di- and Trifluoromethylation of α,β-Unsaturated Carboxylic Acids: A Protocol for Vinylic Fluoroalkylations?[J]. Angewandte Chemie International Edition, 2012, 51 16: 3944-3947. DOI:
10.1002/anie.201200140.[3] DR. ZHENGBIAO HE. Copper-Catalyzed Difluoromethylation of β,γ-Unsaturated Carboxylic Acids: An Efficient Allylic Difluoromethylation?[J]. Angewandte Chemie International Edition, 2012, 51 46: 11545-11547. DOI:
10.1002/anie.201206556.
![3,3-DiMethyl-1-[difluoro(phenylsulfonyl)Methyl]-1,2-benziodoxole Structure](https://www.chemicalbook.com/CAS/20150408/GIF/1052174-67-0.gif)
![3,3-DiMethyl-1-[difluoro(phenylsulfonyl)Methyl]-1,2-benziodoxole 3,3-DiMethyl-1-[difluoro(phenylsulfonyl)Methyl]-1,2-benziodoxole](/NewsImg/2024-01-23/6384160274293716773902182.png)
![3,3-DiMethyl-1-[difluoro(phenylsulfonyl)Methyl]-1,2-benziodoxole 3,3-DiMethyl-1-[difluoro(phenylsulfonyl)Methyl]-1,2-benziodoxole](/NewsImg/2024-01-23/6384160275195768365581876.png)
![3,3-DiMethyl-1-[difluoro(phenylsulfonyl)Methyl]-1,2-benziodoxole 3,3-DiMethyl-1-[difluoro(phenylsulfonyl)Methyl]-1,2-benziodoxole](/NewsImg/2024-01-23/6384160276009714922267705.png)