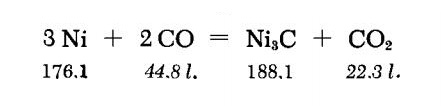Chemical Properties
Nickel carbide is a gray-black powder. Decomposed at room temperature by cone, and dil. Soluble in dil. HNO3; dil. H2SO4 causes separation of C. Stable at temperatures up to 380-400℃. Crystal structure: hexagonal close packing of Ni atoms.
Preparation
Pure NiO is reduced with pure H2 at 275-285℃ until constant weight. The fine Ni powder is heated at once with pure CO (completely free of O3) for 260 hours at 270℃. The Ni3C thus produced is pyrophoric. This may be remedied by heating for a long time in O2-free nitrogen at 250℃ and cooling in the N2 stream.